Question: 2. This problem is adapted from the book Process Heat Transfer, by Q. D. Kern (1950), and develops a model to design bitubular heat transfer.
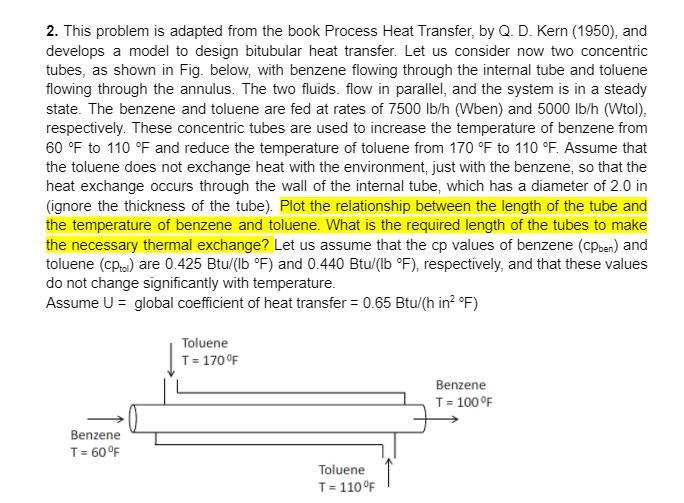
2. This problem is adapted from the book Process Heat Transfer, by Q. D. Kern (1950), and develops a model to design bitubular heat transfer. Let us consider now two concentric tubes, as shown in Fig. below, with benzene flowing through the internal tube and toluene flowing through the annulus. The two fluids, flow in parallel, and the system is in a steady state. The benzene and toluene are fed at rates of 7500 lb/h (Wben) and 5000 lb/h (Wtol), respectively. These concentric tubes are used to increase the temperature of benzene from 60 F to 110 F and reduce the temperature of toluene from 170 F to 110 F. Assume that the toluene does not exchange heat with the environment, just with the benzene, so that the heat exchange occurs through the wall of the internal tube, which has a diameter of 2.0 in (ignore the thickness of the tube). Plot the relationship between the length of the tube and the temperature of benzene and toluene. What is the required length of the tubes to make the necessary thermal exchange? Let us assume that the cp values of benzene (CPben) and toluene (CPtol) are 0.425 Btu/(lb F) and 0.440 Btu/(lb F), respectively, and that these values do not change significantly with temperature. Assume U = global coefficient of heat transfer = 0.65 Btu/(hin? F) Toluene T = 170F Benzene T = 100 F Benzene T = 60F Toluene T = 1100F
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


