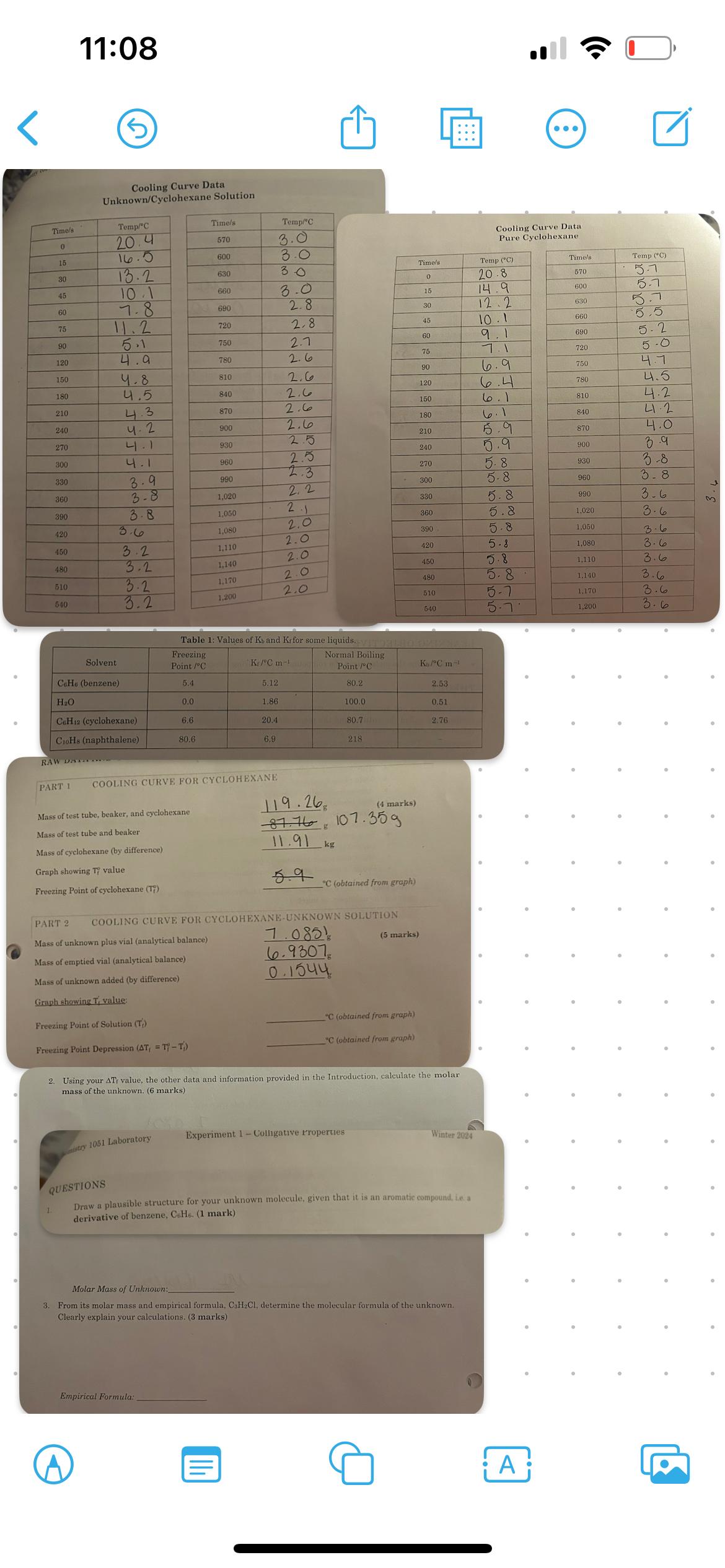
Question: 2 . Use your delta Tf value, the other data and information provided, calcukate the molar mass of the unknown. 3 . From its molar
Use your delta Tf value, the other data and information provided, calcukate the molar mass of the unknown.
From its molar mads and empirical formula, CHCl determine the molecular formula of the unknown. Clearly explain your calculations.
Draw a plausible structure for your unknown molecule, given that it is an aromatic compound, ie a derivative of benzene, CH
More information is given below in the pictures to help fond the answers
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


