Question: 2. We learned how ion concentration differences between the intracellular and extracellular space determines the cell resting potential. For the sake of simplicity, we only
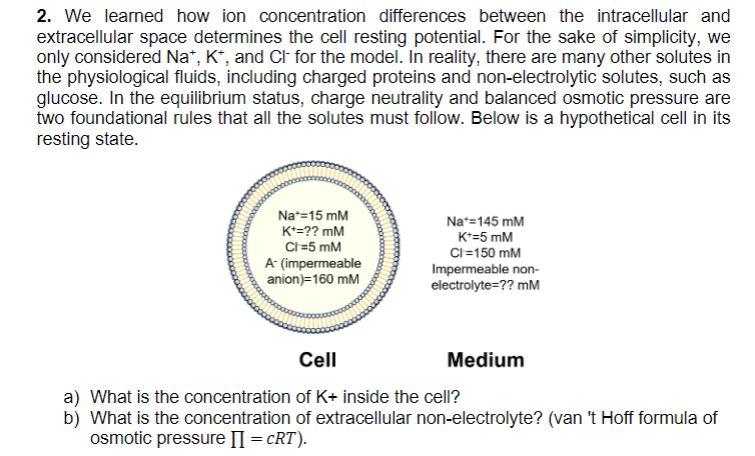
2. We learned how ion concentration differences between the intracellular and extracellular space determines the cell resting potential. For the sake of simplicity, we only considered Na+,K+, and Clfor the model. In reality, there are many other solutes in the physiological fluids, including charged proteins and non-electrolytic solutes, such as glucose. In the equilibrium status, charge neutrality and balanced osmotic pressure are two foundational rules that all the solutes must follow. Below is a hypothetical cell in its resting state. a) What is the concentration of K+ inside the cell? b) What is the concentration of extracellular non-electrolyte? (van 't Hoff formula of osmotic pressure =cRT )
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


