Question: 2. What is the maximum temperature that can be reached by the combustion of one mole of propane (C3H8) with 100% excess air if the
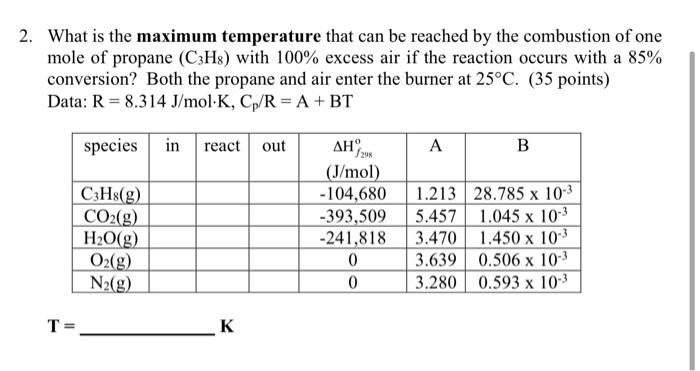
2. What is the maximum temperature that can be reached by the combustion of one mole of propane (C3H8) with 100% excess air if the reaction occurs with a 85% conversion? Both the propane and air enter the burner at 25C. (35 points) Data: R = 8.314 J/mol K, Cp/R = A + BT species in react out A B C3H8(g) CO2(g) H2O(g) O2(g) N2(g) . 29 (J/mol) -104,680 -393,509 -241,818 0 0 1.213 28.785 x 10-3 5.457 1.045 x 103 3.470 1.450 x 103 3.639 0.506 x 10- 3.280 0.593 x 103 T= K 2. What is the maximum temperature that can be reached by the combustion of one mole of propane (C3H8) with 100% excess air if the reaction occurs with a 85% conversion? Both the propane and air enter the burner at 25C. (35 points) Data: R = 8.314 J/mol K, Cp/R = A + BT species in react out A B C3H8(g) CO2(g) H2O(g) O2(g) N2(g) . 29 (J/mol) -104,680 -393,509 -241,818 0 0 1.213 28.785 x 10-3 5.457 1.045 x 103 3.470 1.450 x 103 3.639 0.506 x 10- 3.280 0.593 x 103 T= K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


