Question: 20. Calculate the ionic strength for a solution containing 0.015M NaCl and 0.02M CaSO4. Estimate the activity coefficients for each of the ions (break it
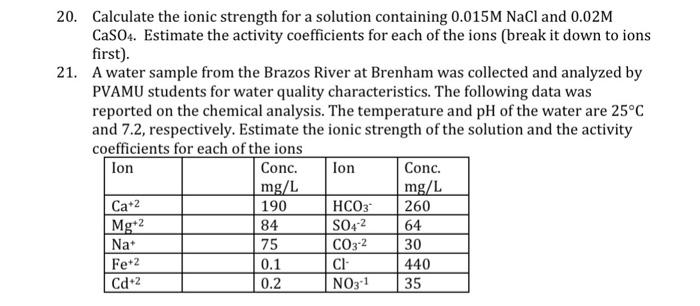
20. Calculate the ionic strength for a solution containing 0.015M NaCl and 0.02M CaSO4. Estimate the activity coefficients for each of the ions (break it down to ions first). 21. A water sample from the Brazos River at Brenham was collected and analyzed by PVAMU students for water quality characteristics. The following data was reported on the chemical analysis. The temperature and pH of the water are 25C and 7.2, respectively. Estimate the ionic strength of the solution and the activity coefficients for each of the ions lon Conc. lon Conc. mg/L mg/L Ca+2 190 HCO3 260 Mgt2 84 S04-2 64 Na 75 CO3-2 30 Fe+2 0.1 CI 440 Cd 2 0.2 NO3-1 35
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


