Question: 21 Answer the following question by either : Handwrite your solution, take a photo/scan, upload/attach the image directly or copy and paste an image of
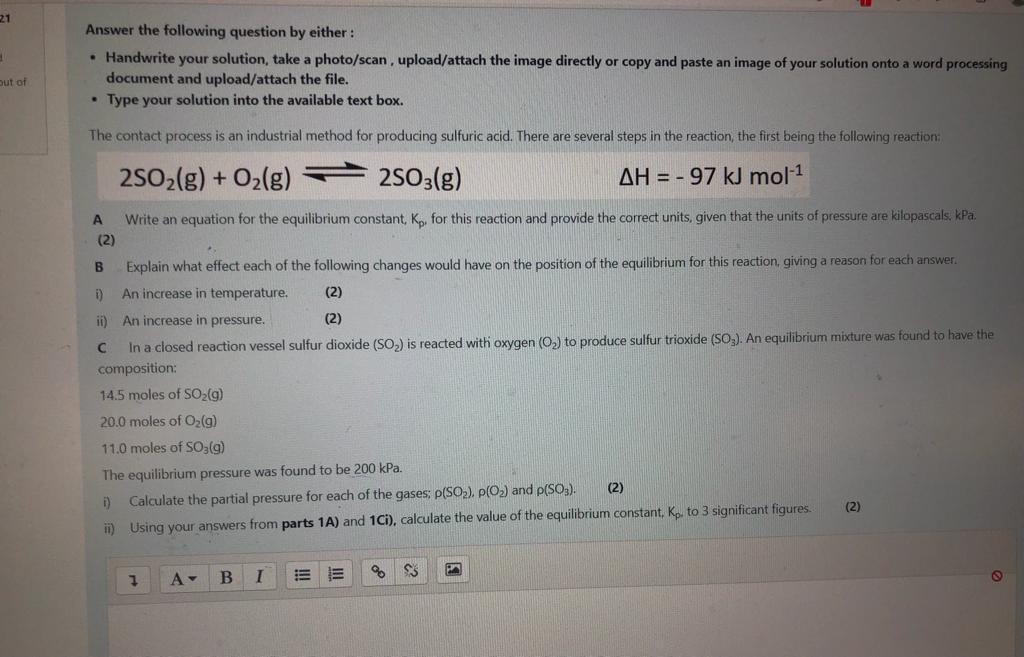
21 Answer the following question by either : Handwrite your solution, take a photo/scan, upload/attach the image directly or copy and paste an image of your solution onto a word processing document and upload/attach the file. Type your solution into the available text box. out of The contact process is an industrial method for producing sulfuric acid. There are several steps in the reaction, the first being the following reaction 2502(g) + O2(g) 2503(g) AH = -97 kJ mol-1 Write an equation for the equilibrium constant, K, for this reaction and provide the correct units, given that the units of pressure are kilopascals. kPa. (2) B Explain what effect each of the following changes would have on the position of the equilibrium for this reaction, giving a reason for each answer. 1) An increase in temperature. (2) ii) An increase in pressure. (2) In a closed reaction vessel sulfur dioxide (SO) is reacted with oxygen (0) to produce sulfur trioxide (SO3). An equilibrium mixture was found to have the composition: 14.5 moles of SO2(g) 20.0 moles of O2(g) 11.0 moles of SO2(g) The equilibrium pressure was found to be 200 kPa. (2) D) Calculate the partial pressure for each of the gases: P(SO2), p(O) and p(SO3). (2) ii) Using your answers from parts 1A) and 1C1), calculate the value of the equilibrium constant, Kp. to 3 significant figures. SS PA 7 A- 1 B
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


