Question: 21 Question 21 (10 points) Using the following conversion factors: 1 mol - MW ing 6.022E23 molecules = 22.414 L of gas at STP How
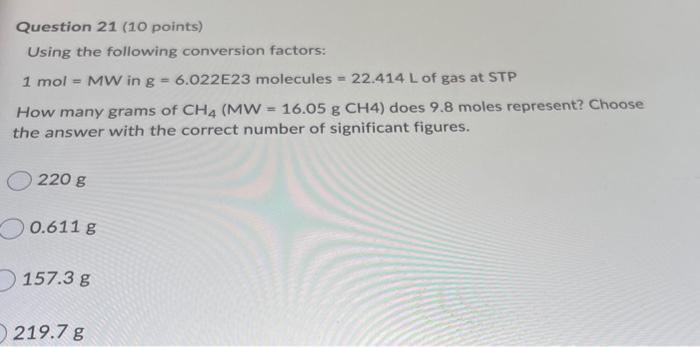

Question 21 (10 points) Using the following conversion factors: 1 mol - MW ing 6.022E23 molecules = 22.414 L of gas at STP How many grams of CH4 (MW = 16.05 g CH4) does 9.8 moles represent? Choose the answer with the correct number of significant figures. 220 g 0.611 g 157.3 g 219.7 g 219.78 O 0.61 g 5.902 x 1024 8 220.8 O g 0.6106 g 05.9 x 1024 g g O 157 g 160 g 5.90 x 1024 g g
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


