Question: 4 Using the following conversion factors: 1 mol = MW in g (2 d.p.) = 6.022E23 molecules = 22.414 L gas at STP How many
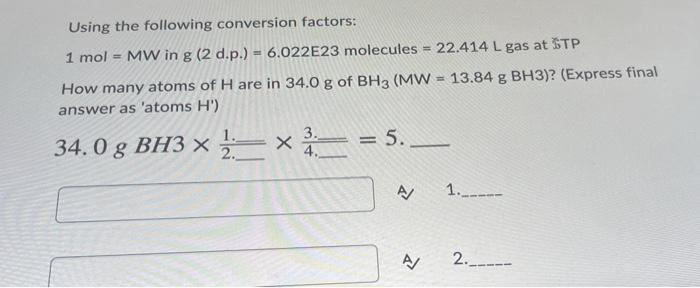
Using the following conversion factors: 1 mol = MW in g (2 d.p.) = 6.022E23 molecules = 22.414 L gas at STP How many atoms of H are in 34.0 g of BH3 (MW = 13.84 g BH3)? (Express final answer as 'atoms H) 1 3._ X = 5 2 4. 34. O g BH3 x 2.x = 5.- A/ 1.____ A / 2.__
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


