Question: 3 . 3 Does O 2 or H 2 O have the higher pressure at the same values of T and ? b a r
Does or have the higher pressure at the same values of and Use Table to predict, without calculation.
Describe a path between points A and in Fig. along which liquid and gas can be distinguished.
Give physical arguments explaining why the critical pressure and temperature should increase with increasing van der Waals a values.
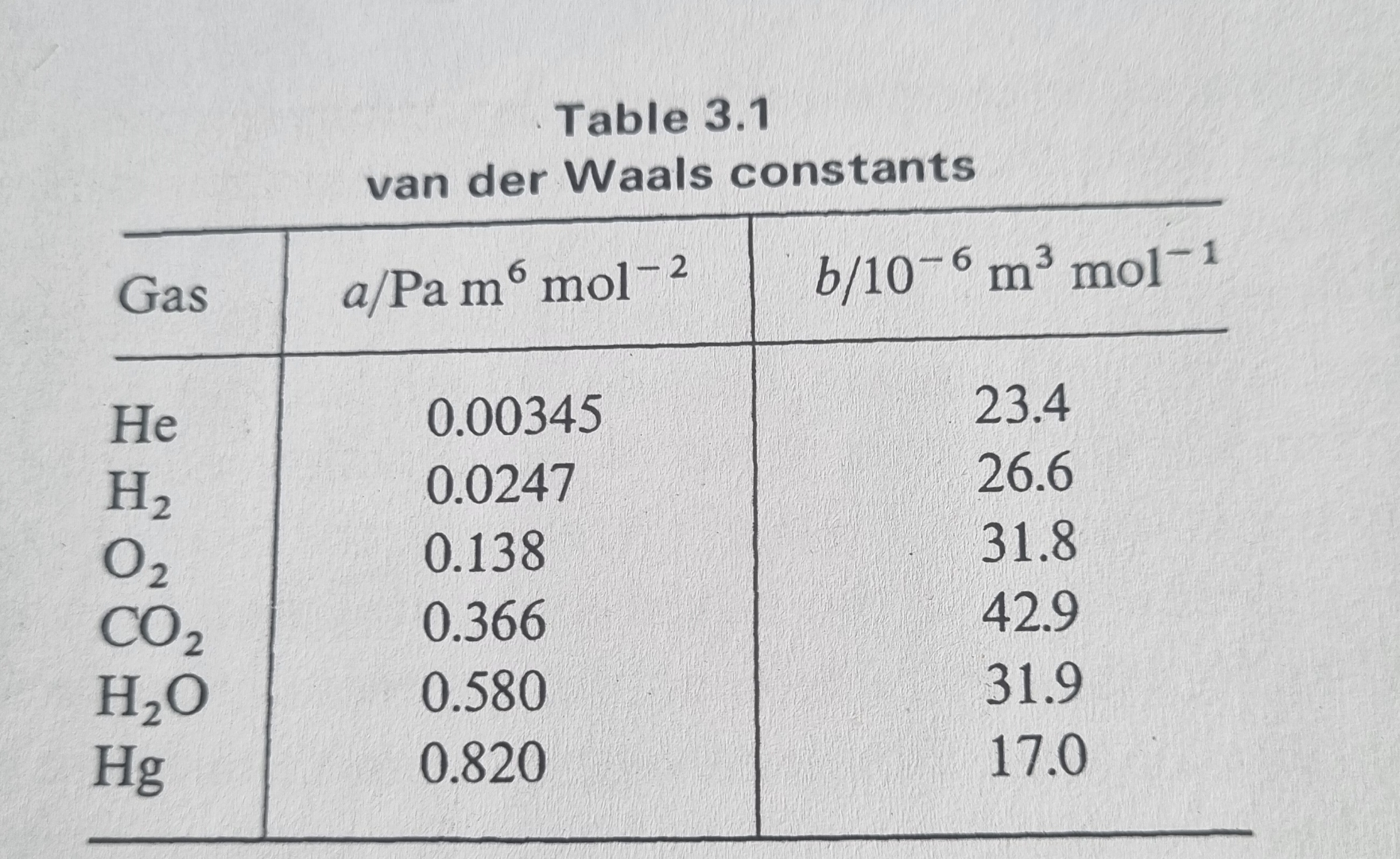
Table
van der Waals constants
table
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


