Question: 3. Calculating the Reaction Rate Constant in Ionic Solution. The reaction rate constant for the reaction between persulfate ions and iodide ions in aqueous solution
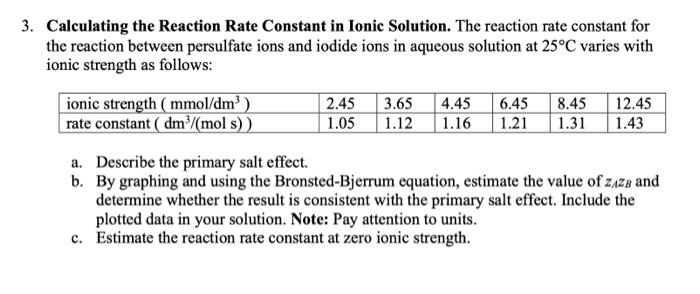
3. Calculating the Reaction Rate Constant in Ionic Solution. The reaction rate constant for the reaction between persulfate ions and iodide ions in aqueous solution at 25C varies with ionic strength as follows: ionic strength (mmol/dm) rate constant ( dm /(mol s)) 2.45 3.65 1.05 1.12 4.45 1.16 6.45 1.21 8.45 1.31 12.45 1.43 a. Describe the primary salt effect. b. By graphing and using the Bronsted-Bjerrum equation, estimate the value of zazz and determine whether the result is consistent with the primary salt effect. Include the plotted data in your solution. Note: Pay attention to units. c. Estimate the reaction rate constant at zero ionic strength
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


