Question: 3) Consider the liquid-phase organic esterification reaction taking place in a CSTR depicted below. Two streams, an acid stream containing no base and a base
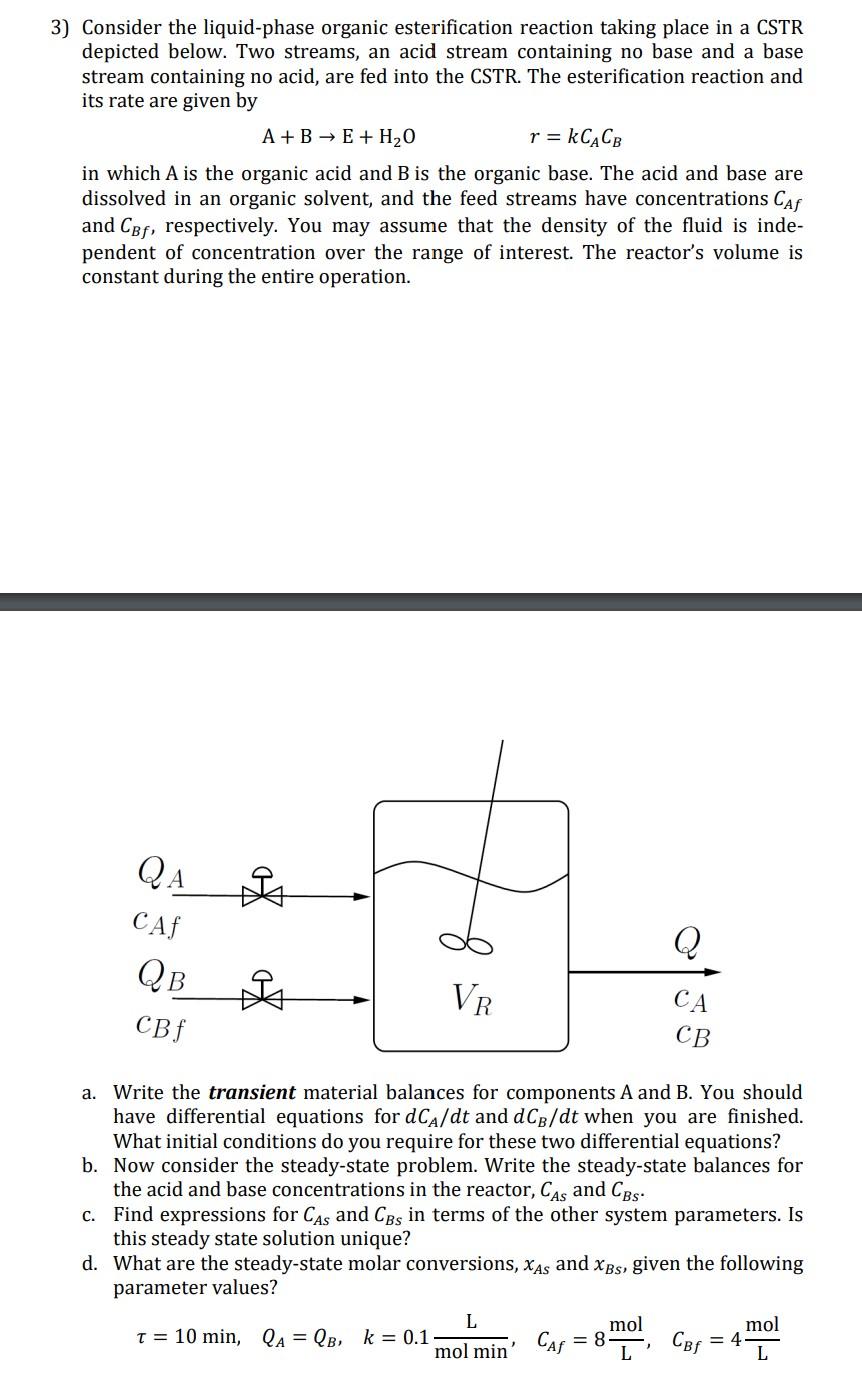
3) Consider the liquid-phase organic esterification reaction taking place in a CSTR depicted below. Two streams, an acid stream containing no base and a base stream containing no acid, are fed into the CSTR. The esterification reaction and its rate are given by A+BE+H2O r = kCACB in which A is the organic acid and B is the organic base. The acid and base are dissolved in an organic solvent, and the feed streams have concentrations Caf and CBf, respectively. You may assume that the density of the fluid is inde- pendent of concentration over the range of interest. The reactor's volume is constant during the entire operation. QA G & CAf QB f Q VR CA CB a. Write the transient material balances for components A and B. You should have differential equations for dCA/dt and dCp/dt when you are finished. What initial conditions do you require for these two differential equations? b. Now consider the steady-state problem. Write the steady-state balances for the acid and base concentrations in the reactor, CAs and CBs. C. Find expressions for CAs and CBs in terms of the other system parameters. Is this steady state solution unique? d. What are the steady-state molar conversions, xas and Xbs, given the following parameter values? L mol t = 10 min, la = QB, k = 0.1 Cof = 4 L L mol 3 mol min' CAF = 8
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


