Question: 3. Consider the process shown in the below figure. A bulk gas stream containing 0.10 mole% of carbon monoxide (CO) gas, 2.0 mole% O2 gas
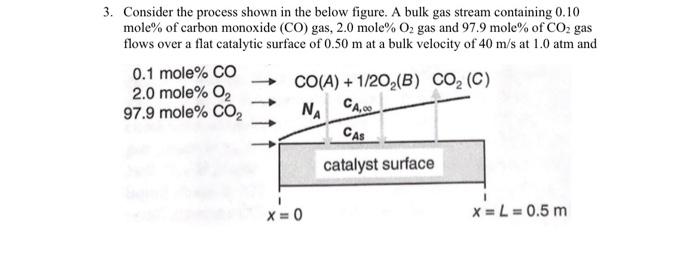
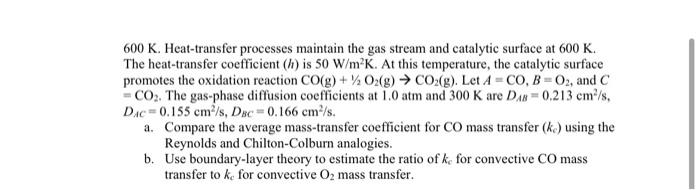
3. Consider the process shown in the below figure. A bulk gas stream containing 0.10 mole% of carbon monoxide (CO) gas, 2.0 mole% O2 gas and 97.9 mole% of CO2 gas flows over a flat catalytic surface of 0.50 m at a bulk velocity of 40 m/s at 1.0 atm and 0.1 mole% CO 2.0 mole% O2 CO(A) + 1/2O2(B) CO2 (C) 97.9 mole% CO2 CAS catalyst surface NA CA, X=0 X = L = 0.5 m 600 K. Heat-transfer processes maintain the gas stream and catalytic surface at 600 K. The heat-transfer coefficient (h) is 50 W/mK. At this temperature, the catalytic surface promotes the oxidation reaction CO(g) + O2(g) CO2(g). Let A =CO, B=02, and C = CO2. The gas-phase diffusion coefficients at 1.0 atm and 300 K are D = 0.213 cm/s, Dic=0.155 cm/s, Dsc 0.166 cm/s. a. Compare the average mass-transfer coefficient for CO mass transfer (ko) using the Reynolds and Chilton-Colburn analogies. b. Use boundary-layer theory to estimate the ratio of k for convective CO mass transfer to ke for convective 02 mass transfer
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


