Question: . Consider the process shown in the figure below, where carbon monoxide (CO) gas is being oxidized to carbon dioxide (CO2). This process is similar
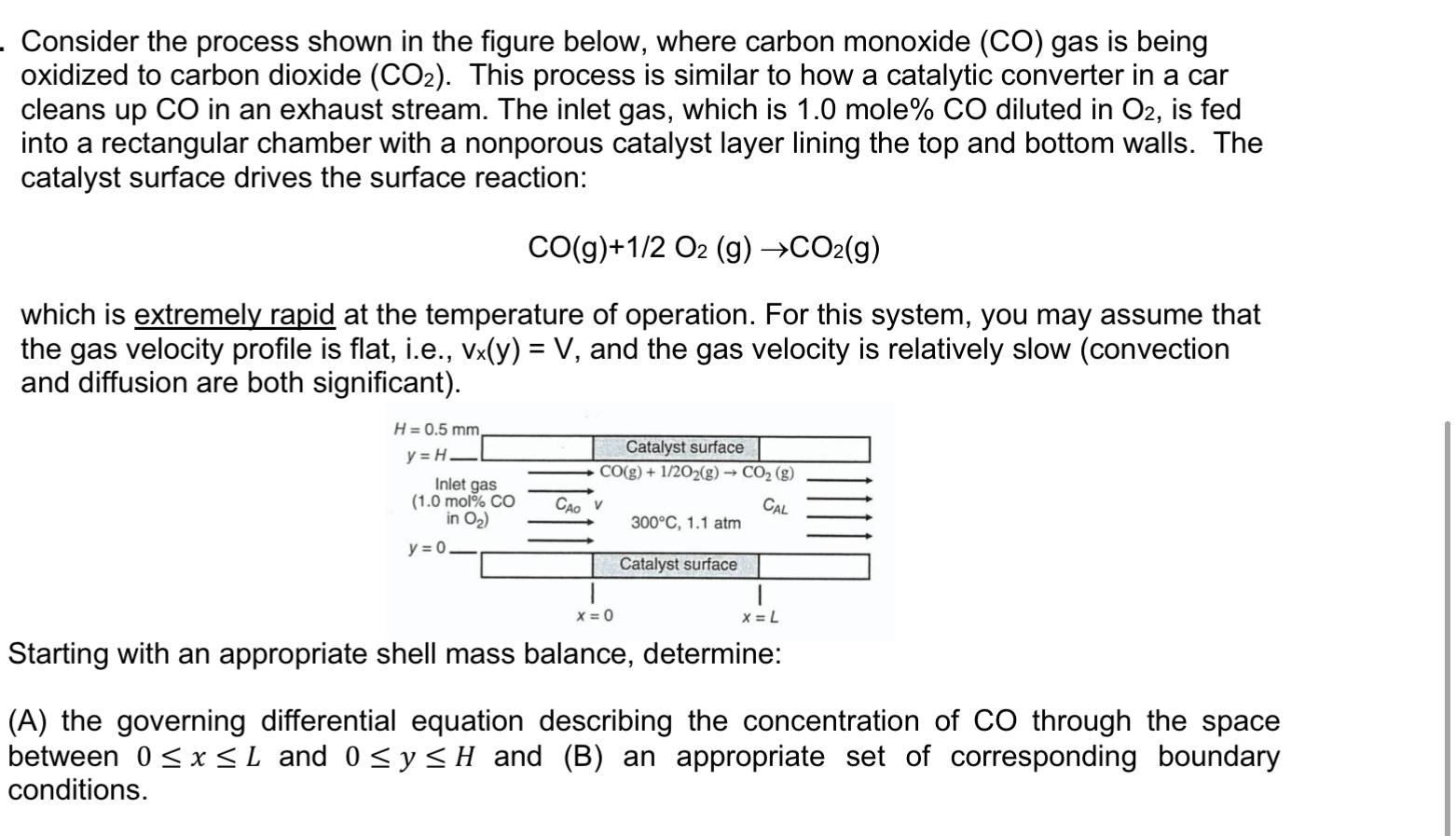
. Consider the process shown in the figure below, where carbon monoxide (CO) gas is being oxidized to carbon dioxide (CO2). This process is similar to how a catalytic converter in a car cleans up CO in an exhaust stream. The inlet gas, which is 1.0 mole% CO diluted in O2, is fed into a rectangular chamber with a nonporous catalyst layer lining the top and bottom walls. The catalyst surface drives the surface reaction: CO(g)+1/2 O2 (g) CO2(g) which is extremely rapid at the temperature of operation. For this system, you may assume that the gas velocity profile is flat, i.e., Vx(y) = V, and the gas velocity is relatively slow (convection and diffusion are both significant). H = 0.5 mm y=H Inlet gas Catalyst surface CO(g) + 1/2O2(g) CO2 (g) CAL 300C, 1.1 atm (1.0 mol% CO in O2) y = 0 Catalyst surface x = 0 X=L Starting with an appropriate shell mass balance, determine: (A) the governing differential equation describing the concentration of co through the space between 0 SX SL and 0 Sy SH and (B) an appropriate set of corresponding boundary conditions
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


