Question: 3 points How Did I Do? Post-Lab Question 12 Dinitrogen monoxide, N_(2)O , decompose into nitrogen N_(2) , and oxygen, O_(2) , when heated. The
3 points\ How Did I Do?\ Post-Lab Question 12\ Dinitrogen monoxide,
N_(2)O, decompose into nitrogen
N_(2), and oxygen,
O_(2), when heated. The initial rate of the reaction is
0.492(M)/(s). What is the initial rate of change for the concentration of
N_(2)i.e.
d(N_(2))/(d)t?\
2N_(2)O(g)->2N_(2)(g)+O_(2)(g)\ Note: Sig figs will be graded.
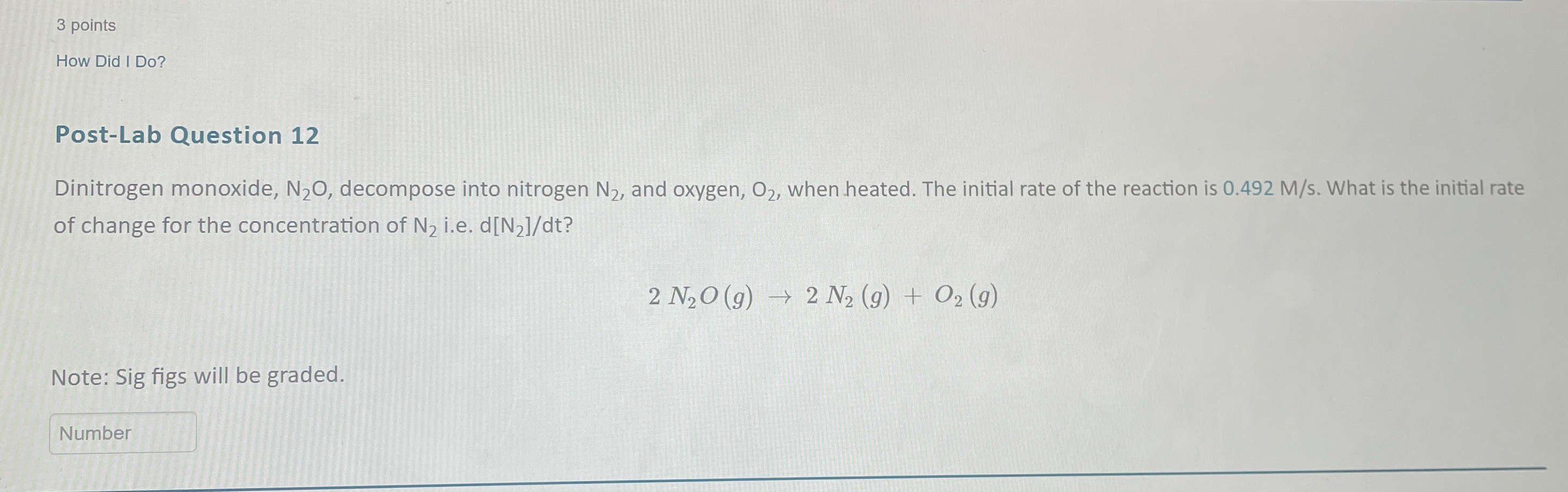
Dinitrogen monoxide, N2O, decompose into nitrogen N2, and oxygen, O2, when heated. The initial rate of the reaction is 0.492M/s. What is the initial rate of change for the concentration of N2 i.e. d[N2]/dt ? 2N2O(g)2N2(g)+O2(g) Note: Sig figs will be graded
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


