Question: 3. The elements sulfur and chlorine react to give sulfur dichloride according to the balanced equation: S8+8Cl28SCl2 Suppose you have 0.6445molS8. (a) How many moles
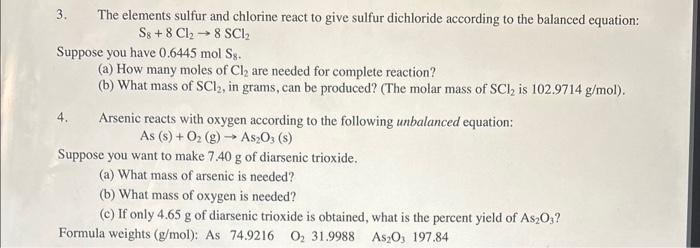
3. The elements sulfur and chlorine react to give sulfur dichloride according to the balanced equation: S8+8Cl28SCl2 Suppose you have 0.6445molS8. (a) How many moles of Cl2 are needed for complete reaction? (b) What mass of SCl2, in grams, can be produced? (The molar mass of SCl2 is 102.9714g/mol ). 4. Arsenic reacts with oxygen according to the following unbalanced equation: As(s)+O2(g)As2O3(s) Suppose you want to make 7.40g of diarsenic trioxide. (a) What mass of arsenic is needed? (b) What mass of oxygen is needed? (c) If only 4.65g of diarsenic trioxide is obtained, what is the percent yield of As2O3 ? Formula weights (g/mol): As 74.9216O231.9988As2O3197.84
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


