Question: 3. Write a balanced i) molecular equation, ii) total ionic equation and iii) net ionic equation to illustrate the following reaction of aluminum bromide and

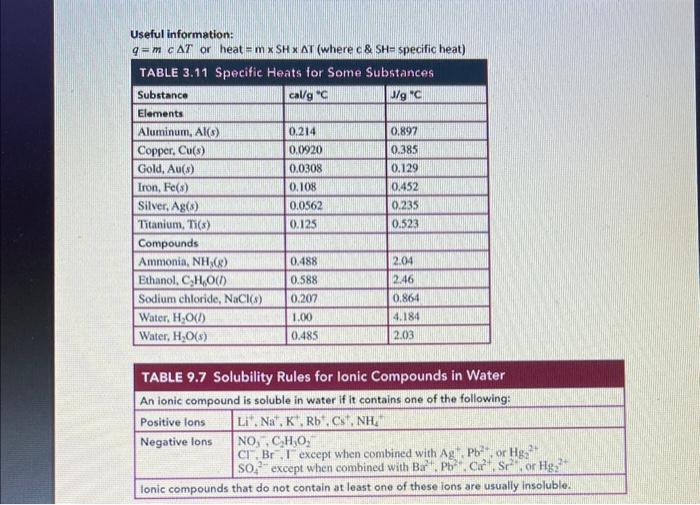
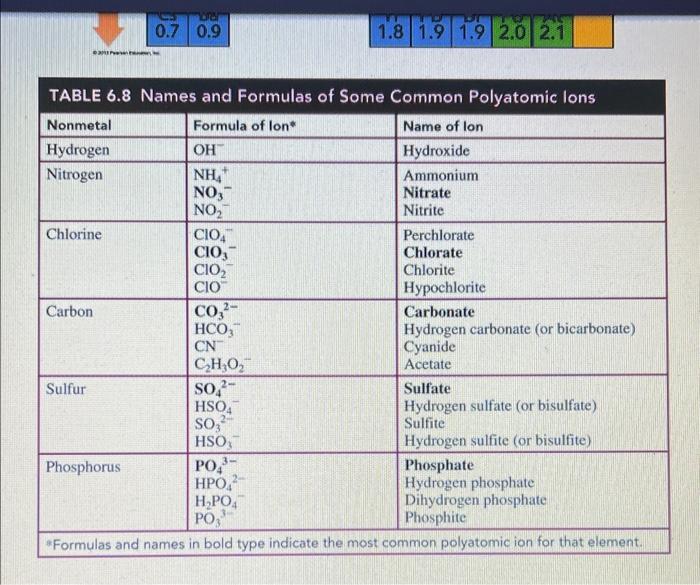
3. Write a balanced i) molecular equation, ii) total ionic equation and iii) net ionic equation to illustrate the following reaction of aluminum bromide and potassium phosphate: (Be sure to include if it is aqueous, a solid, a liquid or a gas). ( 5 marks total) i) balanced molecular equation ( 2 marks) ii) total ionic equation ( 2 marks) iii) net ionic equation ( 1 mark) - do this on a separate piece of paper/tablet and upload a pdf of your response Useful information: q=mcT or heat =mSHT (where c&SH= specific heat) TABLE 6.8 Names and Formulas of Some Common Polyatomic lons
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


