Question: 3)Calculate the time to reach 60% conversion for reactant A in the gas phase reaction AB performed in a batch reactor. The reactor volume is
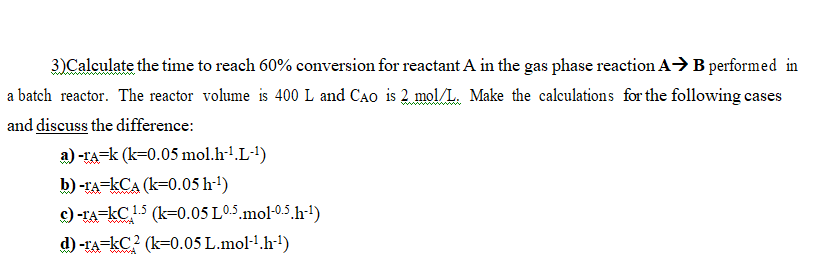
3)Calculate the time to reach 60% conversion for reactant A in the gas phase reaction AB performed in a batch reactor. The reactor volume is 400 L and Cao is 2.mol/L. Make the calculations for the following cases and discuss the difference: a) -ra=k (k=0.05 mol.h-1.L-) b) -ra=kCA (k=0.05 h--) c)-ra=kC 1.5 (k=0.05L05.mol-05.h--) d) -1A=kC2 (k=0.05 L.mol-1.h--)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


