Question: 4 . 1 . Consider the reactor shown in Figure 4 . 1 . The reaction is exothermic. A cooling system is provided to remove
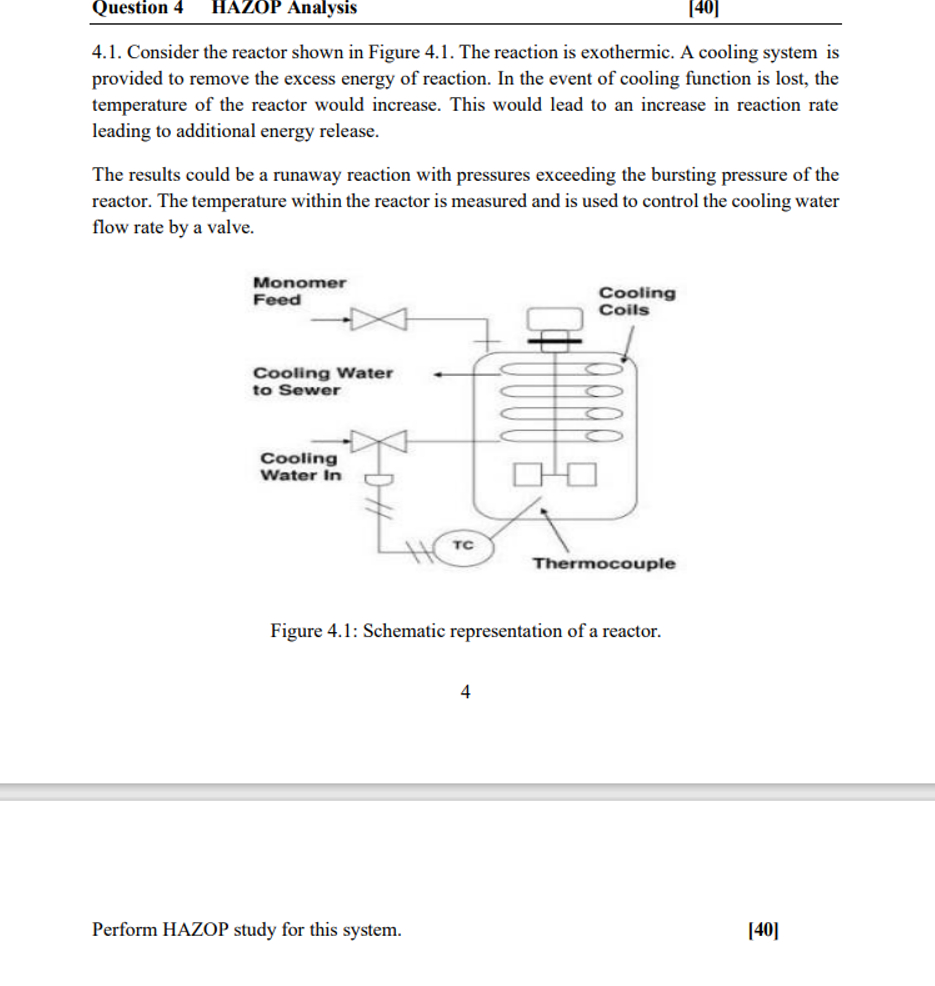
Consider the reactor shown in Figure The reaction is exothermic. A cooling system is
provided to remove the excess energy of reaction. In the event of cooling function is lost, the
temperature of the reactor would increase. This would lead to an increase in reaction rate
leading to additional energy release.
The results could be a runaway reaction with pressures exceeding the bursting pressure of the
reactor. The temperature within the reactor is measured and is used to control the cooling water
flow rate by a valve.
Figure : Schematic representation of a reactor.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


