Question: 4. Given the equation for heat is q=mTC Solve the following : 2.56g of copper was heated from 25.45C to 80.45C. How much energy was
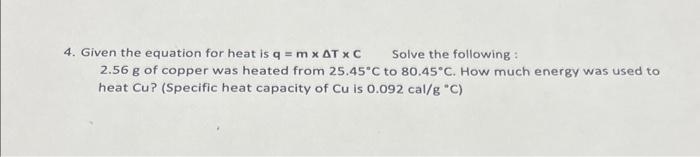
4. Given the equation for heat is q=mTC Solve the following : 2.56g of copper was heated from 25.45C to 80.45C. How much energy was used to heat Cu? (Specific heat capacity of Cu is 0.092cal/g"C )
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


