Question: 4. The curve on the left below is typical for a gas-solid catalytic exothermic reaction carried out adiabatically with a flow rate FAO=4mol/sec. The diagram
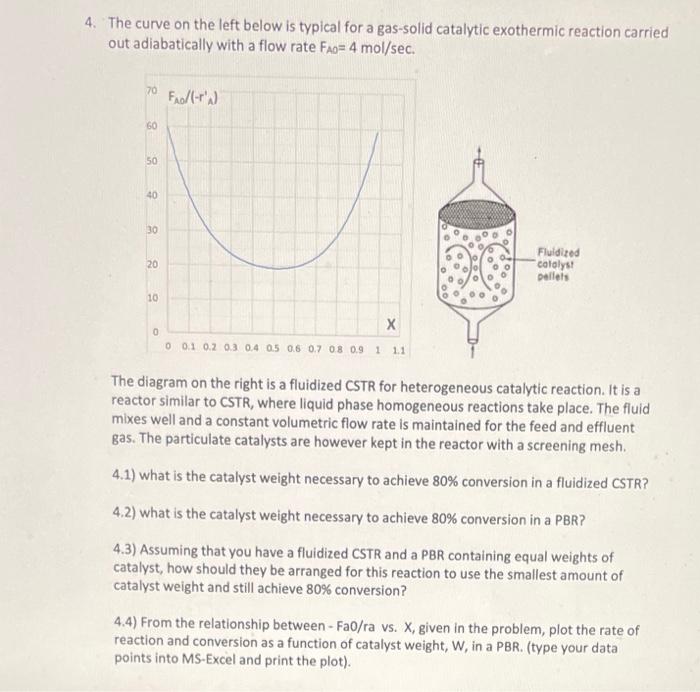
4. The curve on the left below is typical for a gas-solid catalytic exothermic reaction carried out adiabatically with a flow rate FAO=4mol/sec. The diagram on the right is a fluidized CSTR for heterogeneous catalytic reaction. It is a reactor similar to CSTR, where liquid phase homogeneous reactions take place. The fluid mixes well and a constant volumetric flow rate is maintained for the feed and effluent gas. The particulate catalysts are however kept in the reactor with a screening mesh. 4.1) what is the catalyst weight necessary to achieve 80% conversion in a fluidized CSTR? 4.2) what is the catalyst weight necessary to achieve 80% conversion in a PBR? 4.3) Assuming that you have a fluidized CSTR and a PBR containing equal weights of catalyst, how should they be arranged for this reaction to use the smallest amount of catalyst weight and still achieve 80% conversion? 4.4) From the relationship between - Fa0/ra vs. X, given in the problem, plot the rate of reaction and conversion as a function of catalyst weight, W, in a PBR. (type your data points into MS-Excel and print the plot)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


