Question: 4.12. From the van der Waals equation a p= RT V - b 02 and assuming that the molar heat capacity is a constant, C,
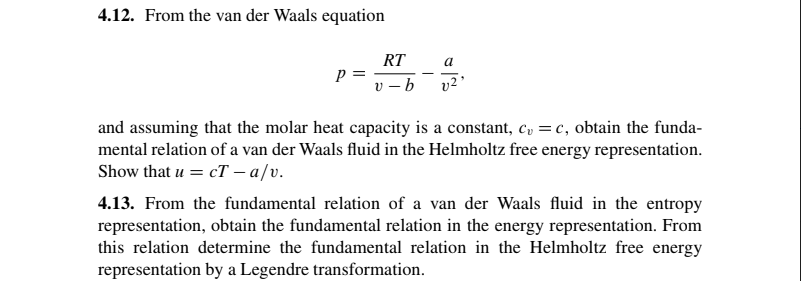
4.12. From the van der Waals equation a p= RT V - b 02 and assuming that the molar heat capacity is a constant, C, =c, obtain the funda- mental relation of a van der Waals fluid in the Helmholtz free energy representation. Show that u = cT a/v. 4.13. From the fundamental relation of a van der Waals fluid in the entropy representation, obtain the fundamental relation in the energy representation. From this relation determine the fundamental relation in the Helmholtz free energy representation by a Legendre transformation
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


