Question: 5. (4 points) When cyclohexane is reacted with bromine in carbon tetrachloride, which product(s) are produced? III. A mixture of I and II 6. (4
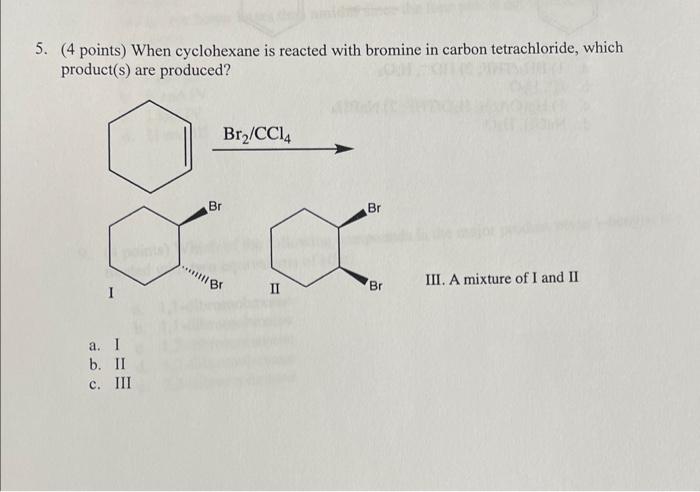

5. (4 points) When cyclohexane is reacted with bromine in carbon tetrachloride, which product(s) are produced? III. A mixture of I and II 6. (4 points) What is an explanation for your choice to the reaction in the previous question? a. The two bromines add simultaneously to the same side of the molecule (syn addition) b. A cyclic bromonium ion is formed which then undergoes SN2 attack to give only the trans product. c. A carbocation intermediate is formed which undergoes attack equally from either side so both isomers are formed
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


