Question: (5) (7 pts total) When we use the Nernst equation to predict equilibrium values, we use the original ion concentrations. Why is a correction not

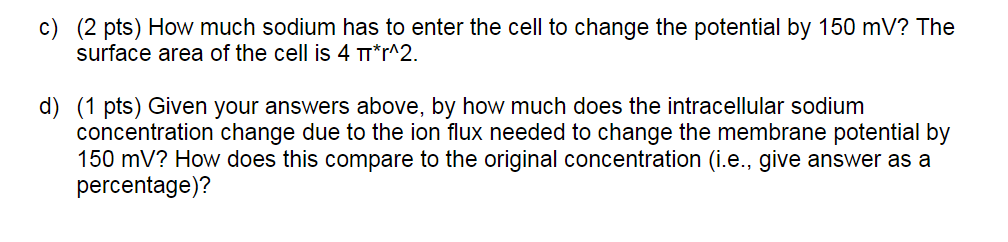
(5) (7 pts total) When we use the Nernst equation to predict equilibrium values, we use the original ion concentrations. Why is a correction not needed to account for the change in concentration due to the ions that flow during the time that the membrane potential is changing? The reason is that the lipid bilayer is an excellent insulator, so few charges have to move to establish a relatively large potential difference. The exact number can be calculated by the following steps. c) (2 pts) How much sodium has to enter the cell to change the potential by 150 mV? The surface area of the cell is 4 m*r^2. d) (1 pts) Given your answers above, by how much does the intracellular sodium concentration change due to the ion flux needed to change the membrane potential by 150 mV? How does this compare to the original concentration (i.e., give answer as a percentage)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


