Question: 5. Use the standard half-cell potentials listed below to calculate the standard cell potential for the following reaction occurring in an electrochemical cell at 25
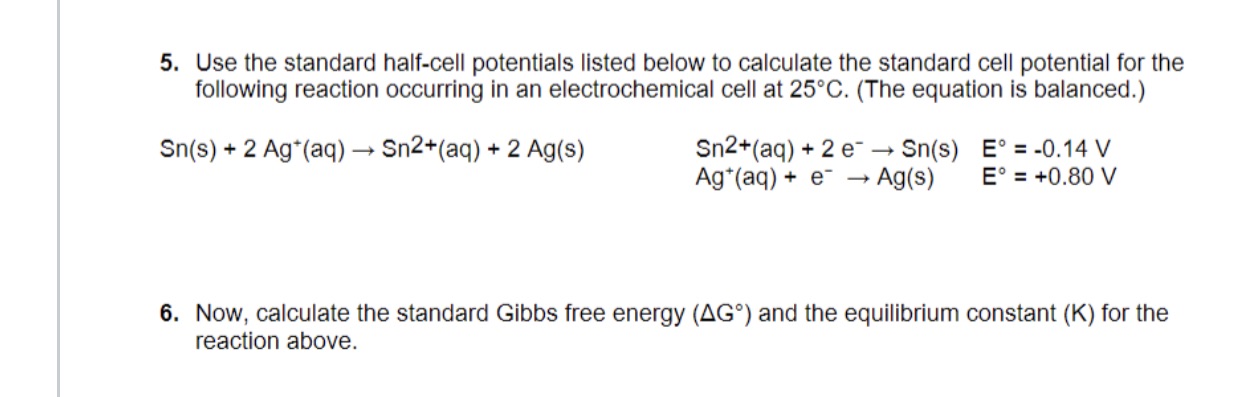
5. Use the standard half-cell potentials listed below to calculate the standard cell potential for the following reaction occurring in an electrochemical cell at 25 C. (The equation is balanced.) Sn(s) + 2 Ag*(aq) - Sn2+(aq) + 2 Ag(s) Sn2+(aq) + 2 e- - Sn(S) E =-0.14 V Ag*(aq) + e- - Ag(S) E. = +0.80 V 6. Now, calculate the standard Gibbs free energy (AG.) and the equilibrium constant (K) for the reaction above
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


