Question: 6. Moving bed is used in catalytic reactors continuously to remove and replace spent catalyst. One example is to produce xylene from toluene disproportionation.
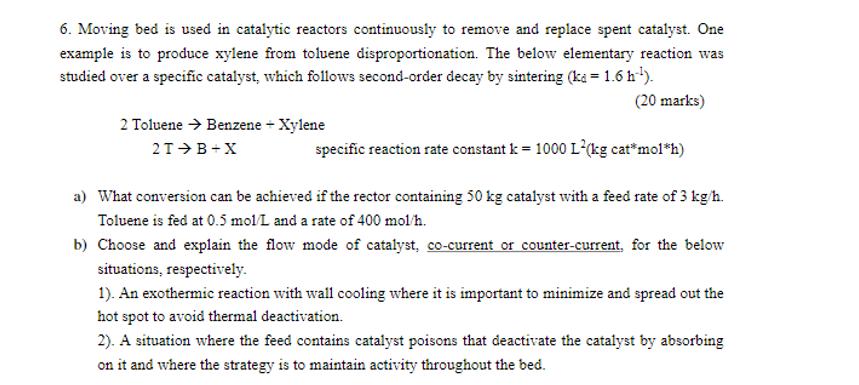
6. Moving bed is used in catalytic reactors continuously to remove and replace spent catalyst. One example is to produce xylene from toluene disproportionation. The below elementary reaction was studied over a specific catalyst, which follows second-order decay by sintering (ka = 1.6 h-). (20 marks) specific reaction rate constant k = 1000 L(kg cat*mol*h) a) What conversion can be achieved if the rector containing 50 kg catalyst with a feed rate of 3 kg/h. Toluene is fed at 0.5 mol/L and a rate of 400 mol/h. 2 Toluene Benzene + Xylene 2T B+X b) Choose and explain the flow mode of catalyst, co-current or counter-current, for the below situations, respectively. 1). An exothermic reaction with wall cooling where it is important to minimize and spread out the hot spot to avoid thermal deactivation. 2). A situation where the feed contains catalyst poisons that deactivate the catalyst by absorbing on it and where the strategy is to maintain activity throughout the bed.
Step by Step Solution
3.56 Rating (146 Votes )
There are 3 Steps involved in it
a To determine the conversion that can be achieved in the reactor we need to first determine the reaction rate of the toluene disproportionation react... View full answer

Get step-by-step solutions from verified subject matter experts


