Question: 6 Please try the problem again, your previous tries are listed below. A solution of Chlorine (net Chloride) and Carbon disulfide, (CS 2 ) has
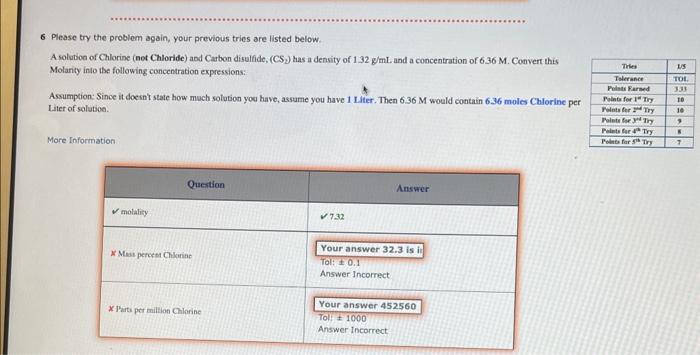
6 Please try the problem again, your previous tries are listed below. A solution of Chlorine (net Chloride) and Carbon disulfide, (CS 2 ) has a density of 1.32g/mL and a concentration of 636M. Convert this Molarity into the following concentration expressions: Assumption: Since it doesnt state how moch solution you have, assume you have 1 Lifer. Then 6.36M would contain 6.36 moles Chlorine pe Liter of solution. More Information
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


