Question: 6.13 Consider a constant-volume continuous stirred tank reactor (CSTR) shown in Figure P6.13. Cro CR CP Figure P.6.13. The following reactions are taking place in
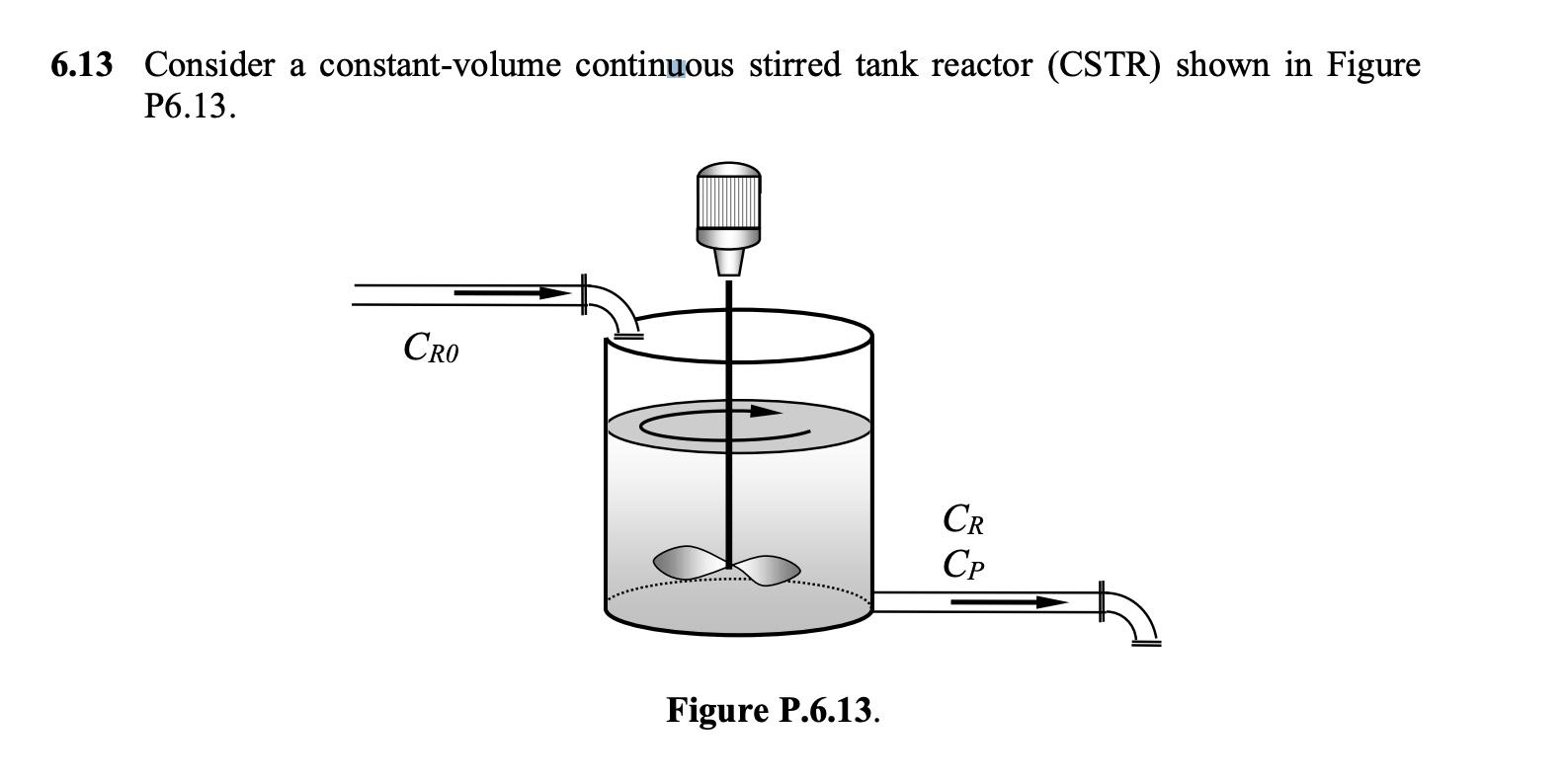
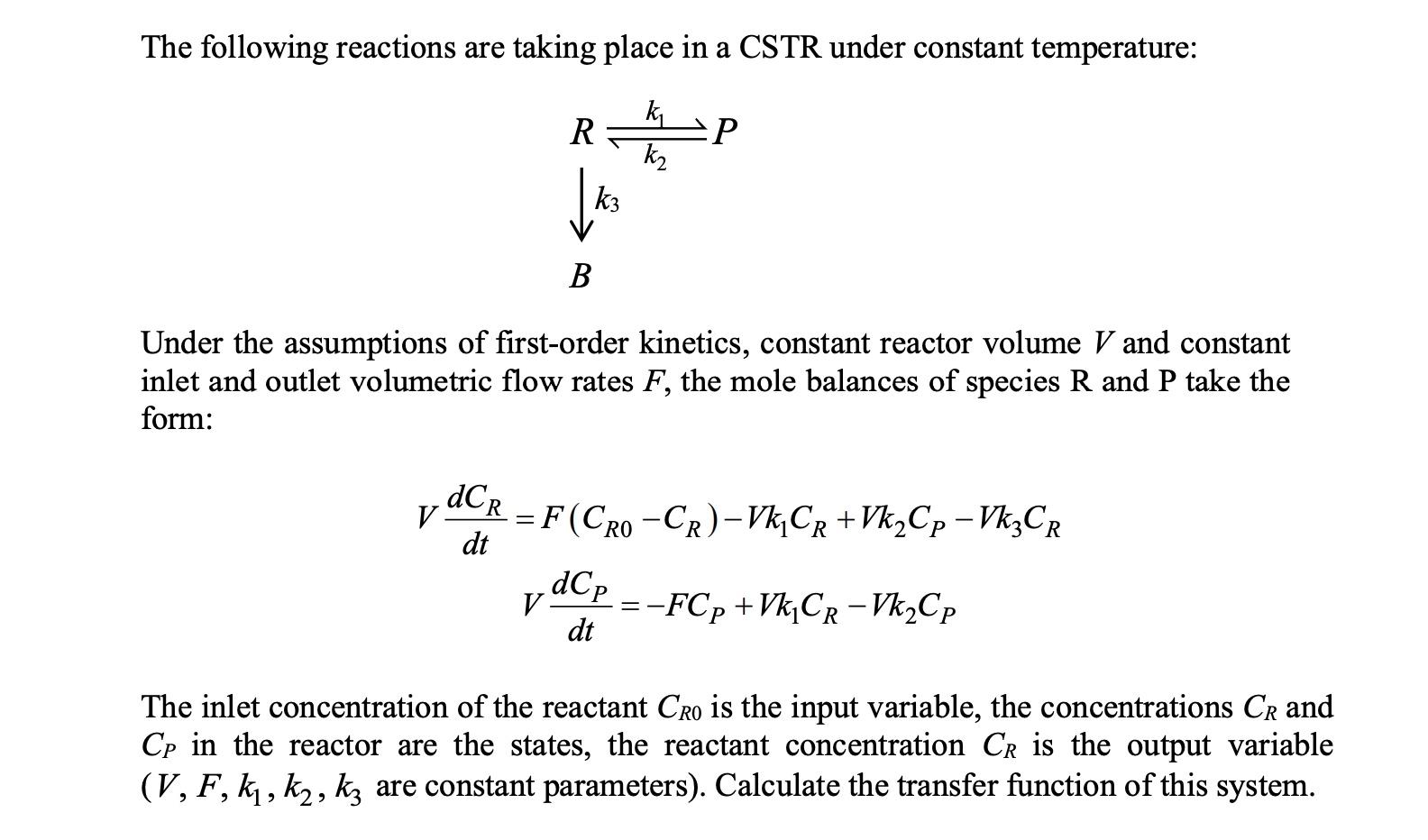
6.13 Consider a constant-volume continuous stirred tank reactor (CSTR) shown in Figure P6.13. Cro CR CP Figure P.6.13. The following reactions are taking place in a CSTR under constant temperature: a R k2 V k3 B Under the assumptions of first-order kinetics, constant reactor volume V and constant inlet and outlet volumetric flow rates F, the mole balances of species R and P take the form: V DCR = F (Cro -CR)-Vk CR+Vk2Cp - Vk2CR RO dt dCp V dt - :-FCp +VkCR-Vk2Cp The inlet concentration of the reactant Cro is the input variable, the concentrations Cr and Cp in the reactor are the states, the reactant concentration CR is the output variable (V, F, kj, k2, kz are constant parameters). Calculate the transfer function of this system. 9 2 >
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


