Question: Consider a constant-volume continuous stirred tank reactor (CSTR) shown in Figure P6.6. CRO Figure P6.6 The following reaction takes place isothermally in the CSTR:
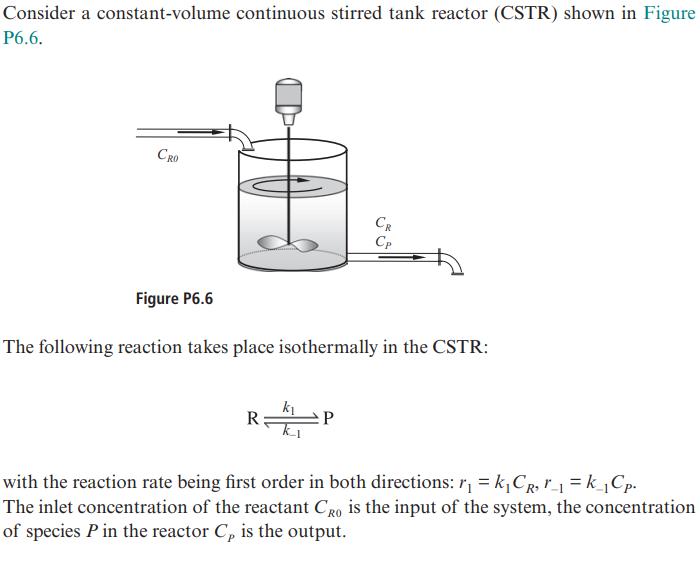
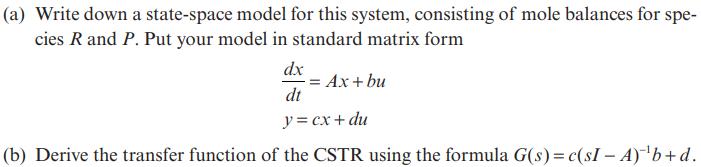
Consider a constant-volume continuous stirred tank reactor (CSTR) shown in Figure P6.6. CRO Figure P6.6 The following reaction takes place isothermally in the CSTR: R k CR Cp P with the reaction rate being first order in both directions: r = kCR, r=k_Cp. The inlet concentration of the reactant CRO is the input of the system, the concentration of species P in the reactor C, is the output. (a) Write down a state-space model for this system, consisting of mole balances for spe- cies R and P. Put your model in standard matrix form dx -= Ax + bu dt y = cx + du (b) Derive the transfer function of the CSTR using the formula G(s)= c(sI - A) b+d.
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


