Question: 8. For simplicity, lone-pair electrons are often left out in the bond-line structure of a molecule. (a) Fill in the lone-pair electrons where needed in
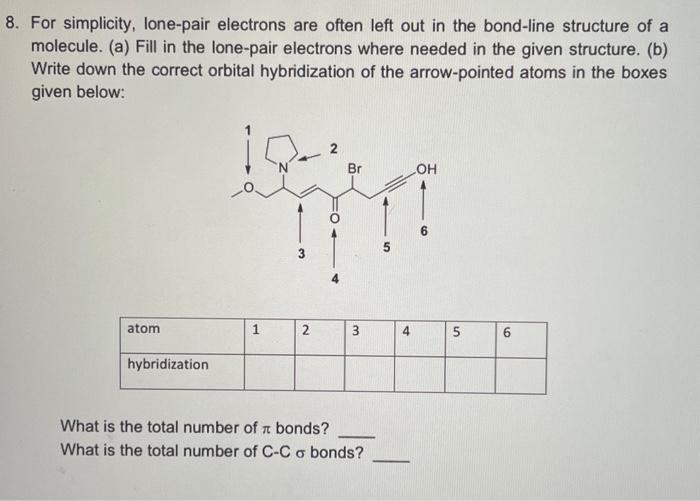
8. For simplicity, lone-pair electrons are often left out in the bond-line structure of a molecule. (a) Fill in the lone-pair electrons where needed in the given structure. (b) Write down the correct orbital hybridization of the arrow-pointed atoms in the boxes given below: What is the total number of bonds? What is the total number of CC bonds
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


