Question: 9. (3 pts) Using the solubility product, Ksp,, explain why, in lime-soda softening, that: i. calcium is removed from water as a carbonate solid, rather
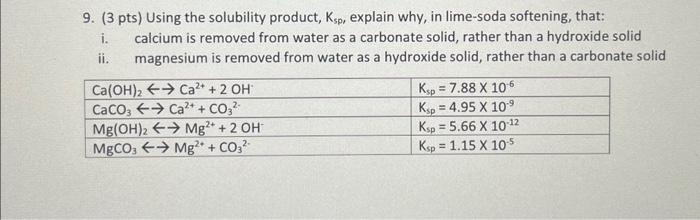
9. (3 pts) Using the solubility product, Ksp,, explain why, in lime-soda softening, that: i. calcium is removed from water as a carbonate solid, rather than a hydroxide solid ii. magnesium is removed from water as a hydroxide solid, rather than a carbonate solid
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


