Question: A 0.194g sample of an unknown solid is dissolved in 9.82g of cyclohexane. The change in the freezing point of the solution is 2.94 C.
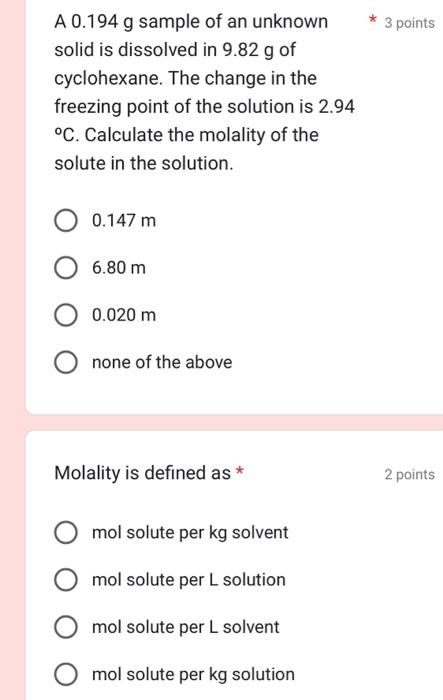
A 0.194g sample of an unknown solid is dissolved in 9.82g of cyclohexane. The change in the freezing point of the solution is 2.94 C. Calculate the molality of the solute in the solution. 0.147m6.80m0.020m none of the above Molality is defined as * 2 poin mol solute per kg solvent mol solute per L solution mol solute per L solvent mol solute per kg solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


