Question: a 34. Consider the reaction: N2(g) + O2(g) + 2 NO 0.25 mol of nitrogen and 0.25 mol of oxygen are placed in a 5.00
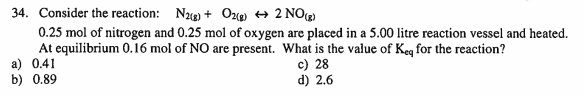
a 34. Consider the reaction: N2(g) + O2(g) + 2 NO 0.25 mol of nitrogen and 0.25 mol of oxygen are placed in a 5.00 litre reaction vessel and heated. At equilibrium 0.16 mol of NO are present. What is the value of Key for the reaction? a) 0.41 c) 28 b) 0.89 d) 2.6
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


