Question: a. A polymer electrolyte membrane fuel cell (PEMFC) operates at 80C on pure hydrogen (1atm) supplied on the anode side and air (1atm) on the
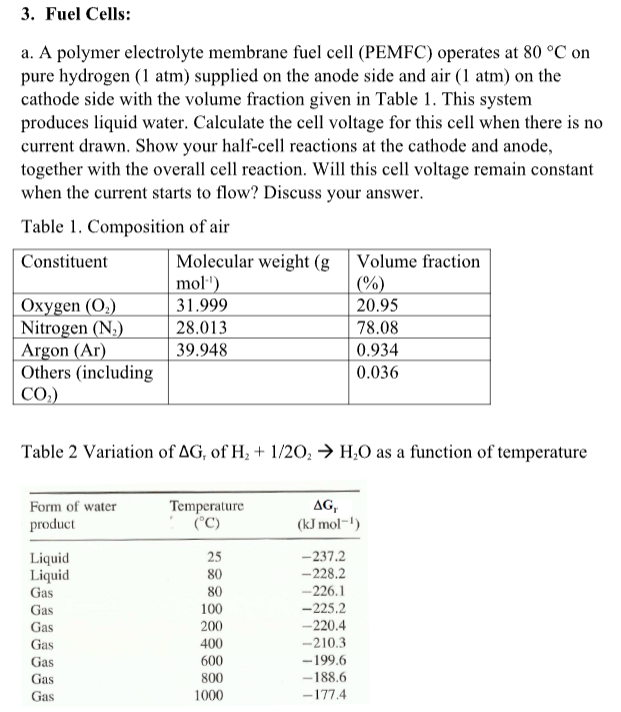
a. A polymer electrolyte membrane fuel cell (PEMFC) operates at 80C on pure hydrogen (1atm) supplied on the anode side and air (1atm) on the cathode side with the volume fraction given in Table 1. This system produces liquid water. Calculate the cell voltage for this cell when there is no current drawn. Show your half-cell reactions at the cathode and anode, together with the overall cell reaction. Will this cell voltage remain constant when the current starts to flow? Discuss your answer. Table 1. Composition of air Table 2 Variation of Gr of H2+1/2O2H2O as a function of temperature
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


