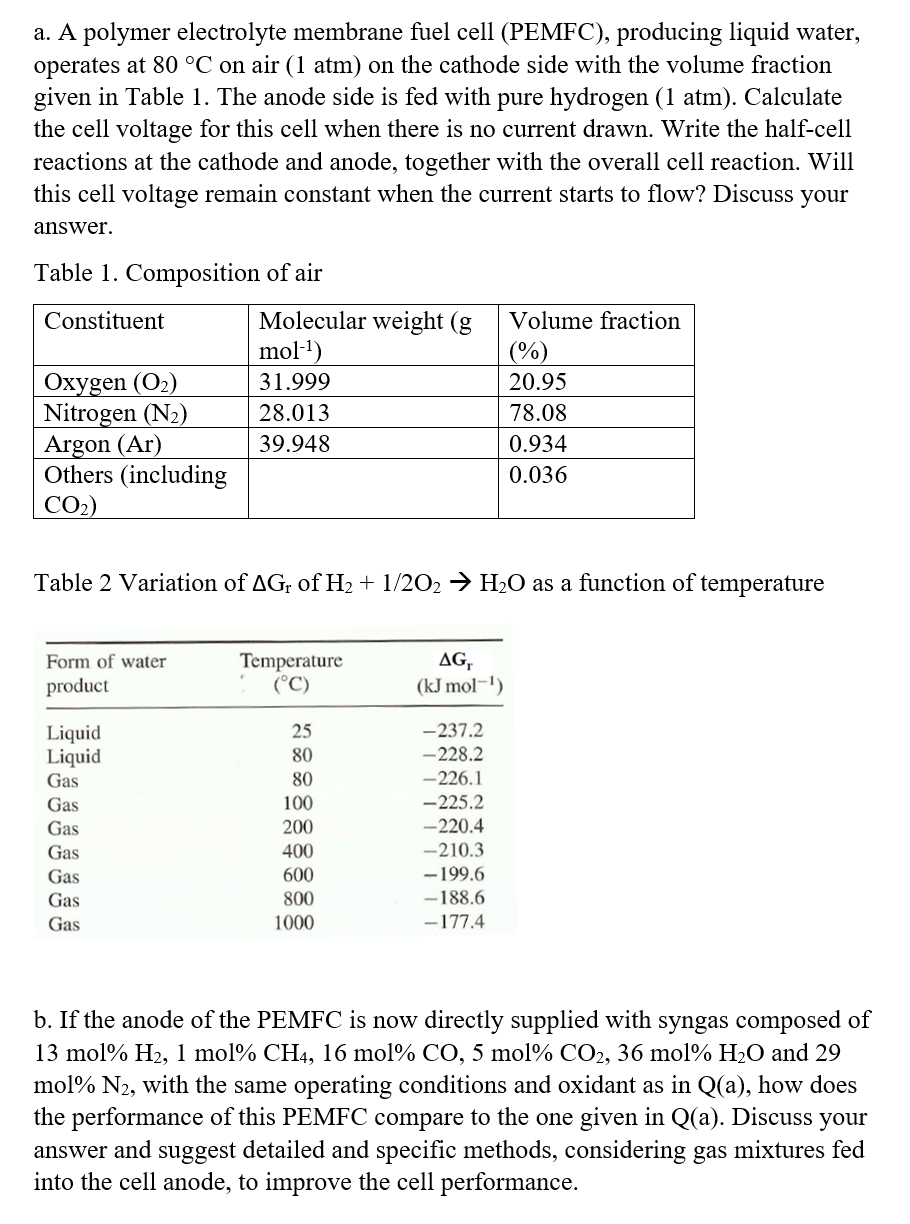
Question: a . A polymer electrolyte membrane fuel cell ( PEMFC ) , producing liquid water, operates at 8 0 C on air ( 1 atm
a A polymer electrolyte membrane fuel cell PEMFC producing liquid water, operates at on air atm on the cathode side with the volume fraction given in Table The anode side is fed with pure hydrogen atm. Calculate the cell voltage for this cell when there is no current drawn. Write the halfcell reactions at the cathode and anode, together with the overall cell reaction. Will this cell voltage remain constant when the current starts to flow? Discuss your answer.
Table Composition of air
Table Variation of of as a function of temperature
b If the anode of the PEMFC is now directly supplied with syngas composed of
molmolmolmolmol and
mol with the same operating conditions and oxidant as in how does
the performance of this PEMFC compare to the one given in Qa Discuss your
answer and suggest detailed and specific methods, considering gas mixtures fed
into the cell anode, to improve the cell performance.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


