Question: a) An acid-base titration experiment was conducted by using Nitric acid, HNO3 and sodium hydroxide, NaOH. Determine the pH when 50mL of 0.1MHNO3 is titrated
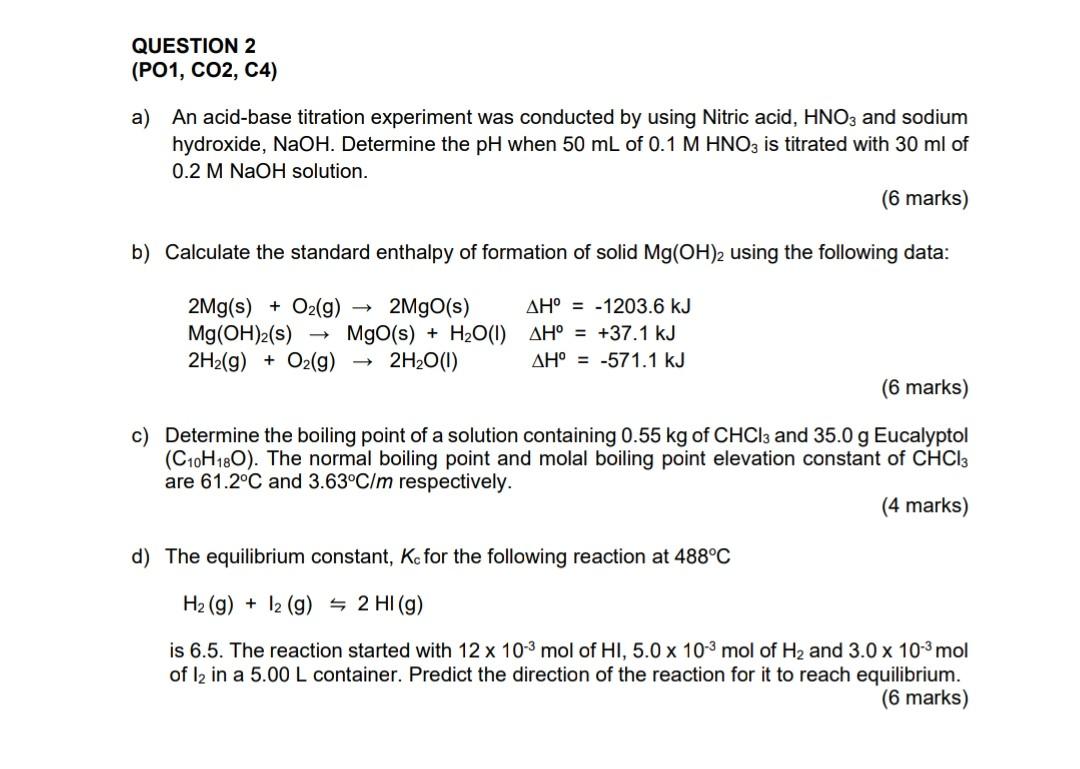
a) An acid-base titration experiment was conducted by using Nitric acid, HNO3 and sodium hydroxide, NaOH. Determine the pH when 50mL of 0.1MHNO3 is titrated with 30ml of 0.2MNaOH solution. (6 marks) b) Calculate the standard enthalpy of formation of solid Mg(OH)2 using the following data: 2Mg(s)+O2(g)2MgO(s)Mg(OH)2(s)MgO(s)+H2O(l)2H2(g)+O2(g)2H2O(l)H0=1203.6kJH=+37.1kJH0=571.1kJ (6 marks) c) Determine the boiling point of a solution containing 0.55kg of CHCl3 and 35.0g Eucalyptol (C10H18O). The normal boiling point and molal boiling point elevation constant of CHCl3 are 61.2C and 3.63C/m respectively. (4 marks) d) The equilibrium constant, Kc for the following reaction at 488C H2(g)+I2(g)2HI(g) is 6.5. The reaction started with 12103mol of HI,5.0103molOH2 and 3.0103mol of I2 in a 5.00L container. Predict the direction of the reaction for it to reach equilibrium. (6 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


