Question: A buffer solution is made that is 0.310M in H2CO3 and 0.310M in KHCO3. If Ka1 for H2CO3 is 4.20107, what is the pH of
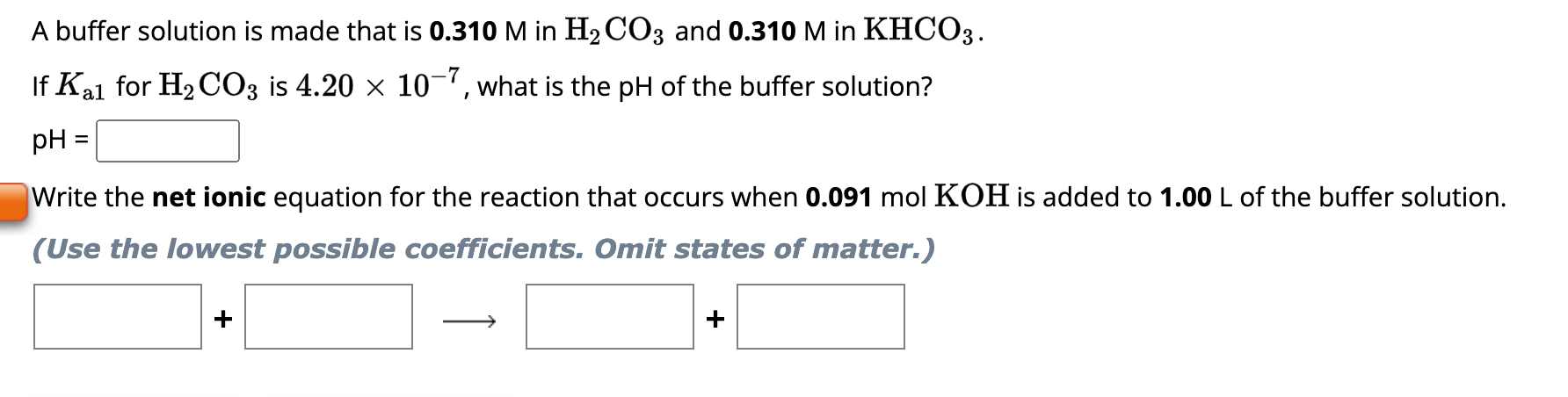
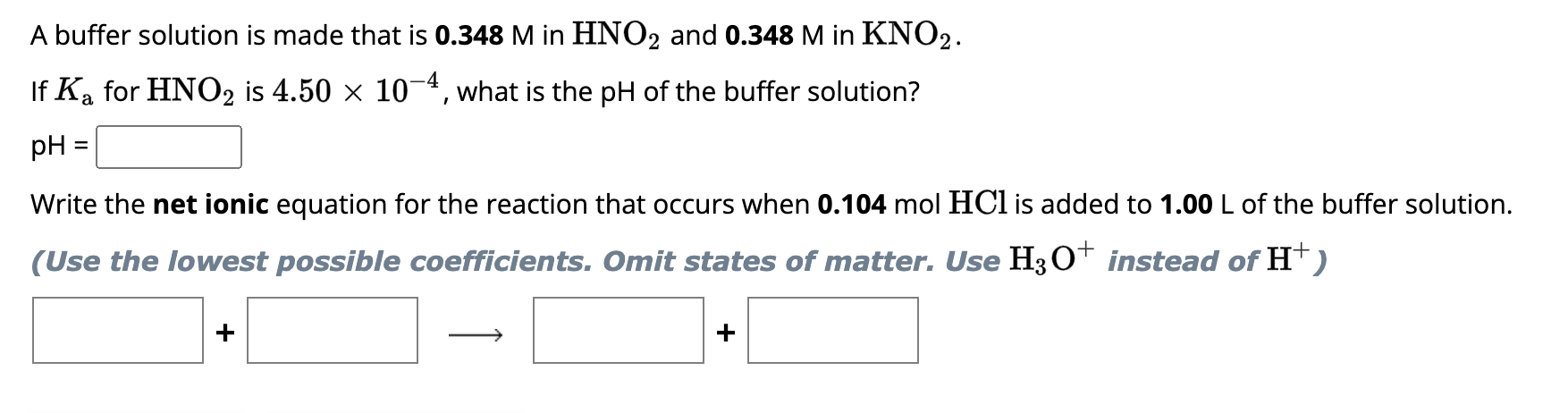
A buffer solution is made that is 0.310M in H2CO3 and 0.310M in KHCO3. If Ka1 for H2CO3 is 4.20107, what is the pH of the buffer solution? pH= Write the net ionic equation for the reaction that occurs when 0.091molKOH is added to 1.00L of the buffer solution. (Use the lowest possible coefficients. Omit states of matter.) + A buffer solution is made that is 0.348M in HNO2 and 0.348M in KNO2. If Ka for HNO2 is 4.50104, what is the pH of the buffer solution? pH= Write the net ionic equation for the reaction that occurs when 0.104molHCl is added to 1.00L of the buffer solution. (Use the lowest possible coefficients. Omit states of matter. Use H3O+instead of H+)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


