Question: (a) Derive the design performance equation for a constant volume batch reactor given i. zero order kinetics ii. first order kinetics iii. second order kinetics
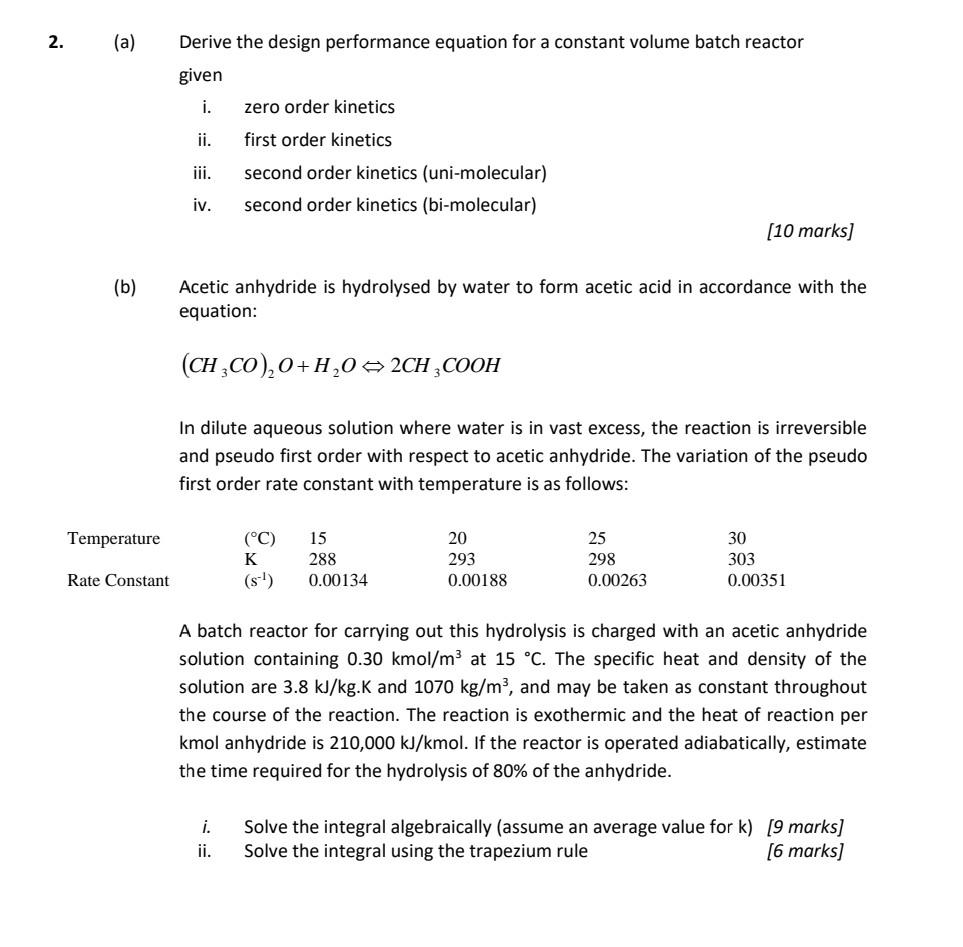
(a) Derive the design performance equation for a constant volume batch reactor given i. zero order kinetics ii. first order kinetics iii. second order kinetics (uni-molecular) iv. second order kinetics (bi-molecular) [10 marks] (b) Acetic anhydride is hydrolysed by water to form acetic acid in accordance with the equation: (CH3CO)2O+H2O2CH3COOH In dilute aqueous solution where water is in vast excess, the reaction is irreversible and pseudo first order with respect to acetic anhydride. The variation of the pseudo first order rate constant with temperature is as follows: A batch reactor for carrying out this hydrolysis is charged with an acetic anhydride solution containing 0.30kmol/m3 at 15C. The specific heat and density of the solution are 3.8kJ/kg.K and 1070kg/m3, and may be taken as constant throughout the course of the reaction. The reaction is exothermic and the heat of reaction per kmol anhydride is 210,000kJ/kmol. If the reactor is operated adiabatically, estimate the time required for the hydrolysis of 80% of the anhydride. i. Solve the integral algebraically (assume an average value for k ) [9 marks] ii. Solve the integral using the trapezium rule [6 marks]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


