Question: a Derive the Nernst Equation and explain the importance. b. A cell is based on the following half-reactions. 3 mks VO+ 2H + VO
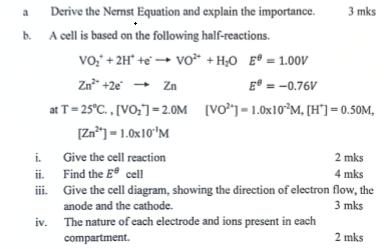
a Derive the Nernst Equation and explain the importance. b. A cell is based on the following half-reactions. 3 mks VO+ 2H + VO + HO E = 1.00V Zn +2e Zn at T=25C., [VO]=2.0M [Zn2]-1.0x10"'M E = -0.76V [VO]-1.0x10M, [H]=0.50M, i. Give the cell reaction 2 mks ii. Find the E cell 4 mks iii. Give the cell diagram, showing the direction of electron flow, the anode and the cathode. 3 mks iv. The nature of each electrode and ions present in each compartment. 2 mks
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


