Question: A first order liquid phase reaction is conducted adiabatically in a CSTR. The feed temperature is 300 K, the mean residence time is 1 h
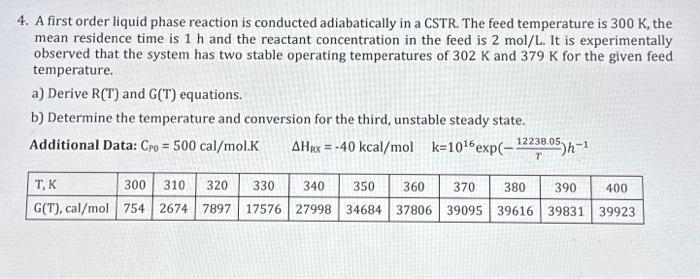
4. A first order liquid phase reaction is conducted adiabatically in a CSTR. The feed temperature is 300K, the mean residence time is 1h and the reactant concentration in the feed is 2mol/L. It is experimentally observed that the system has two stable operating temperatures of 302K and 379K for the given feed temperature. a) Derive R(T) and G(T) equations. b) Determine the temperature and conversion for the third, unstable steady state. Additional Data: CPO=500cal/mol.KHex=40kcal/molk=1016exp(T12238.05)h1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


