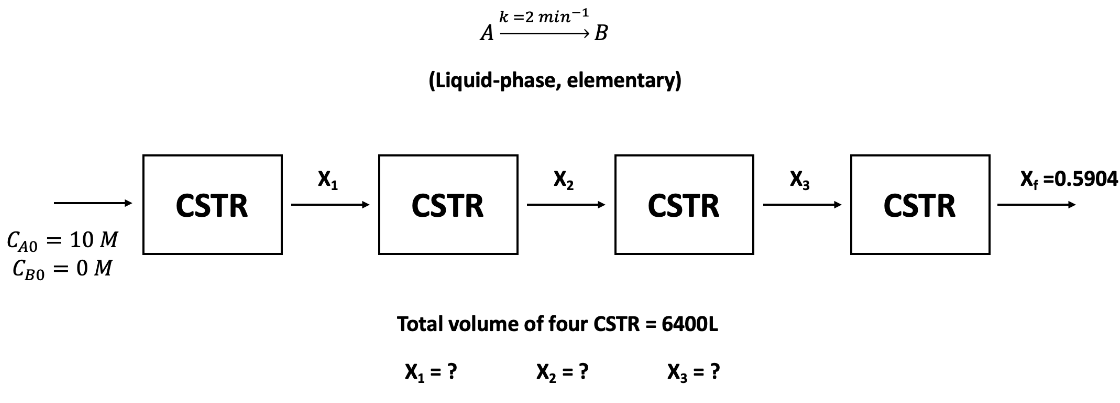
Question: A liquid - phase irreversible reaction: A - > B + S follows the elementary reaction rate law. This reaction is carried out in a
A liquidphase irreversible reaction: ABS follows the elementary reaction rate law. This reaction is carried out in a series of four, equal volume CSTRs at the same reaction temperature. The concentrations of A and B in the feed to the first reactor are CaM and CboM The rate constant k for this reaction is min at the operation temperature. The final conversion is If the total volume of these four reactors is L what is the volume flow rate in Lh to the first reactor? What are the conversions in reactors and X X X Solve by using the "graphing method" r vs C graph
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


