Question: Q2 (8 points ) : ( ) The liquid phase irreversible reaction: AB+C is carried out isothermally in CSTR. To determine rate law for this
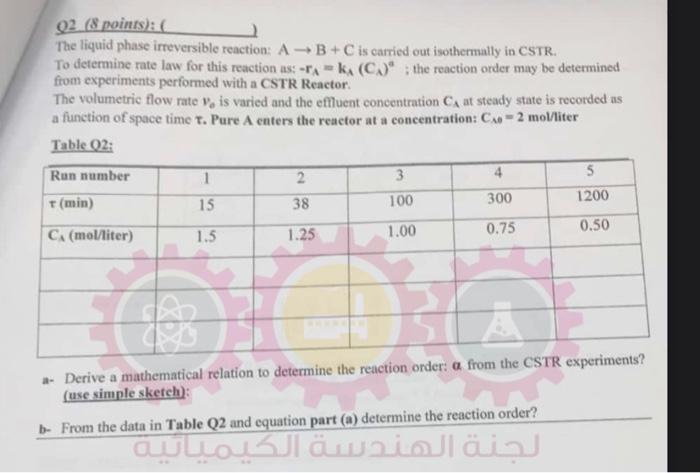
Q2 (8 points ) : ( ) The liquid phase irreversible reaction: AB+C is carried out isothermally in CSTR. To determine rate law for this reaction as: rA=KA(CA)A; the reaction order may be determined from experiments performed with a CSTR Reactor. The volumetric flow rate vb is varied and the effluent concentration CA at steady state is recorded as a function of space time . Pure A enters the reactor at a concentration: CSO=2 mol/iter Table Q2: a- Derive a mathematical relation to determine the reaction order: from the CSTR experimentS? (use simple sketch): b- From the data in Table Q2 and equation part (a) determine the reaction order
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


