Question: A liquid phase reaction, A+BC+D is to be carried out in a wellmixed ideal batch reactor with a constant volume of 10 liters. The initial
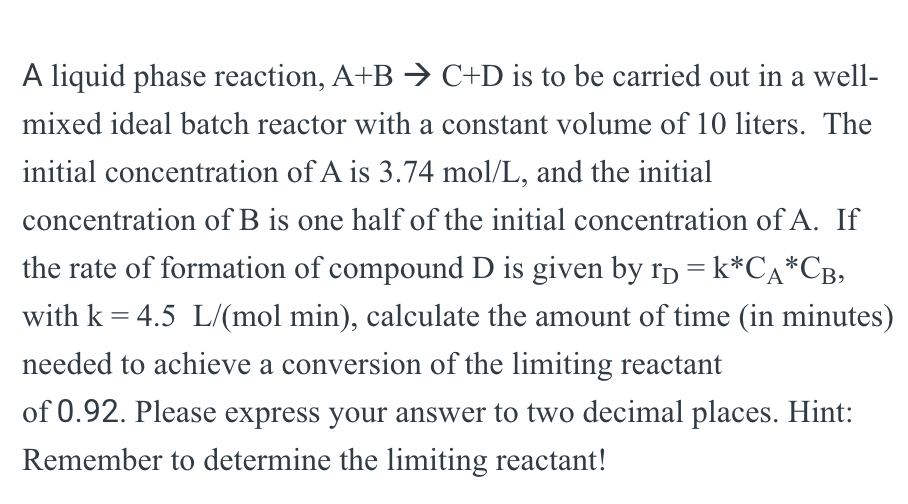
A liquid phase reaction, A+BC+D is to be carried out in a wellmixed ideal batch reactor with a constant volume of 10 liters. The initial concentration of A is 3.74mol/L, and the initial concentration of B is one half of the initial concentration of A. If the rate of formation of compound D is given by rD=kCACB, with k=4.5L/(molmin), calculate the amount of time (in minutes) needed to achieve a conversion of the limiting reactant of 0.92. Please express your answer to two decimal places. Hint: Remember to determine the limiting reactant
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


