Question: A molecule that contains oxygen (O) can have different structures depending on both thermodynamics and kinetic conditions. The following figure, which is from a research
A molecule that contains oxygen (O) can have different structures depending on both thermodynamics and kinetic conditions. The following figure, which is from a research paper, contains the pair correlation functions of oxygen in four different structures (Structures A, B, C, and D indicated by different colors) that are composed of the same type of molecules. Among these four structures, one is in a solid crystalline state, another is in a liquid state, and two others are in different solid amorphous states. Which one of these four structures is in a solid crystalline state? Why? Which one of these four structures is in a liquid state? Why? For the two structures in different solid amorphous states, which one has the higher degrees of disorder? Why?
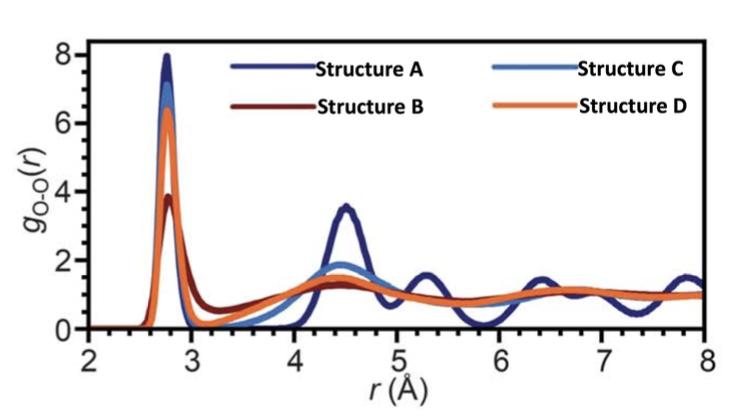
8- -Structure A -Structure B 6. (1)0-06 2 Structure C Structure D 3 4 5 6 7 8 r ()
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


