Question: Problem 3 (20/100): Air is decompressed and heated from its initial state of 5 C and 10 bar to a final state of 60
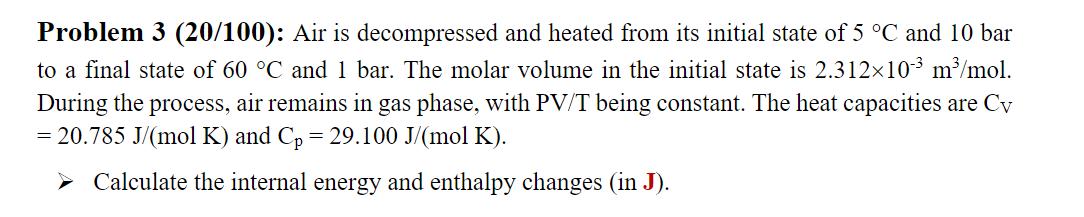
Problem 3 (20/100): Air is decompressed and heated from its initial state of 5 C and 10 bar to a final state of 60 C and 1 bar. The molar volume in the initial state is 2.31210-3 m/mol. During the process, air remains in gas phase, with PV/T being constant. The heat capacities are Cv = 20.785 J/(mol K) and Cp = 29.100 J/(mol K). Calculate the internal energy and enthalpy changes (in J).
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


