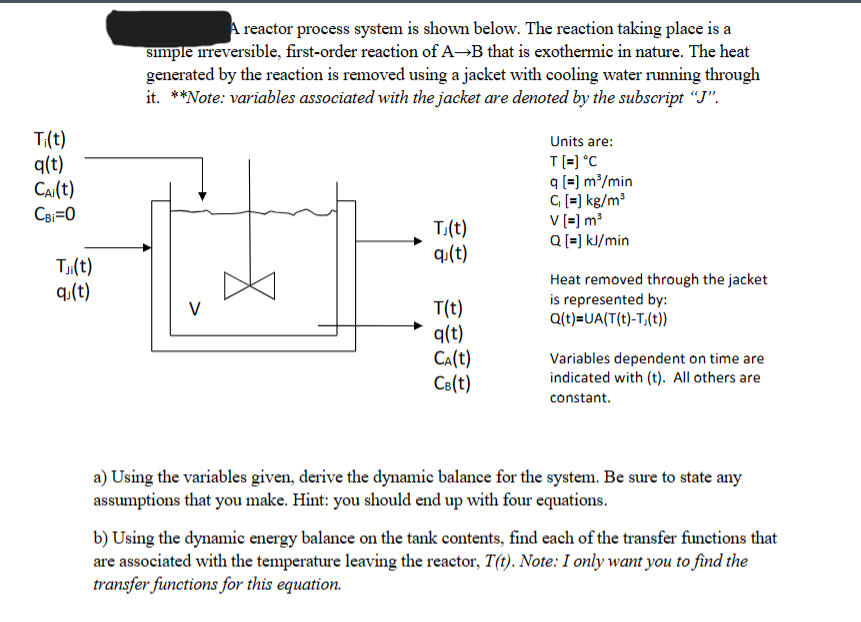
Question: A reactor process system is shown below. The reaction taking place is a simple irreversible, first - order reaction of A B that is exothermic
A reactor process system is shown below. The reaction taking place is a
simple irreversible, firstorder reaction of that is exothermic in nature. The heat
generated by the reaction is removed using a jacket with cooling water running through
itote: variables associated with the jacket are denoted by the subscript
Units are:
Heat removed through the jacket
is represented by:
Variables dependent on time are
indicated with All others are
constant.
a Using the variables given, derive the dynamic balance for the system. Be sure to state any
assumptions that you make. Hint: you should end up with four equations.
b Using the dynamic energy balance on the tank contents, find each of the transfer functions that
are associated with the temperature leaving the reactor, Note: I only want you to find the
transfer functions for this equation.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


