Question: A solution initially contains 2.2 x 10' M in Fe- and 0.33 M in SCN-. If the K, for Fe(SCN)2 2.3 x 10. what concentration
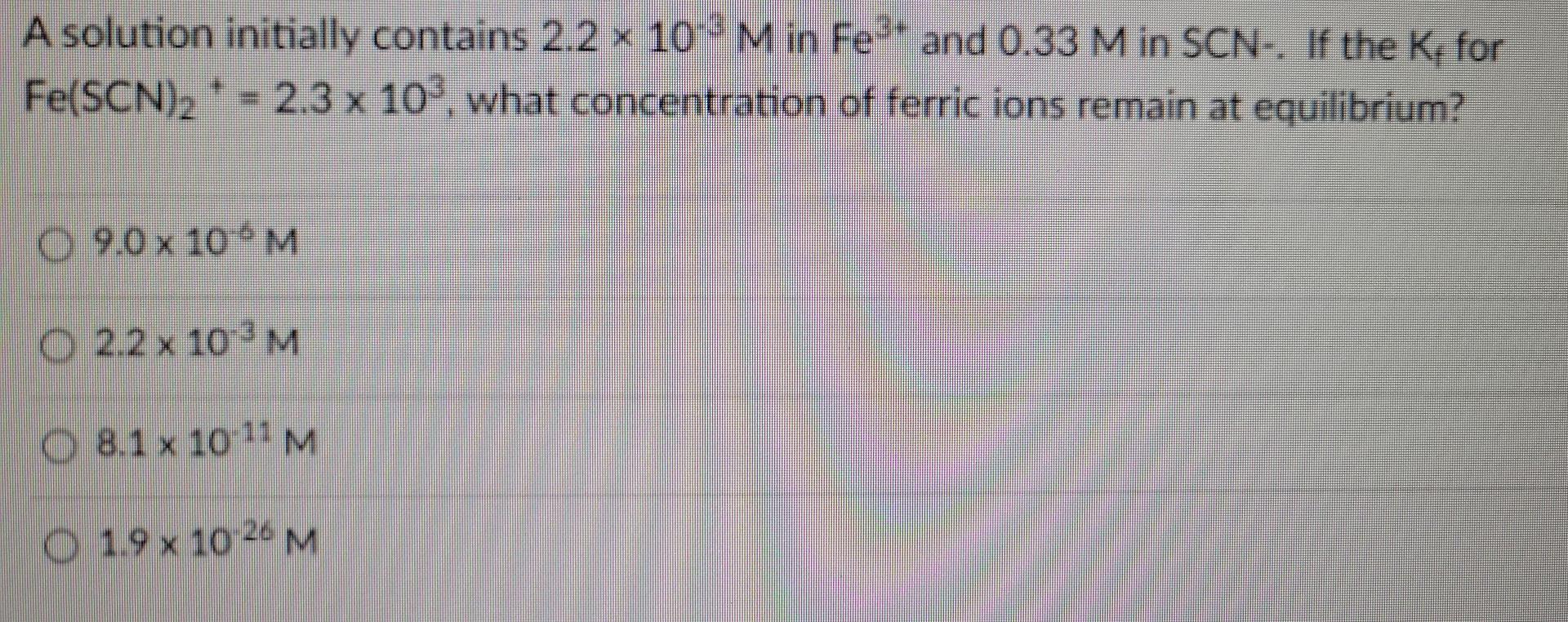
A solution initially contains 2.2 x 10' M in Fe- and 0.33 M in SCN-. If the K, for Fe(SCN)2 2.3 x 10. what concentration of ferric ions remain at equilibrium? O 9.0 x 100M O 2.2 x 10-3M O 8.1 x 10-11 M O 1.9 x 10-26 M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


