Question: A solution is made by adding 0.350 gg Ca(OH)2(s)Ca(OH)2(), 50.0 mLmL of 1.20 MM HNO3HNO3, and enough water to make a final volume of 75.0
A solution is made by adding 0.350 gg Ca(OH)2(s)Ca(OH)2(), 50.0 mLmL of 1.20 MM HNO3HNO3, and enough water to make a final volume of 75.0 mLmL.
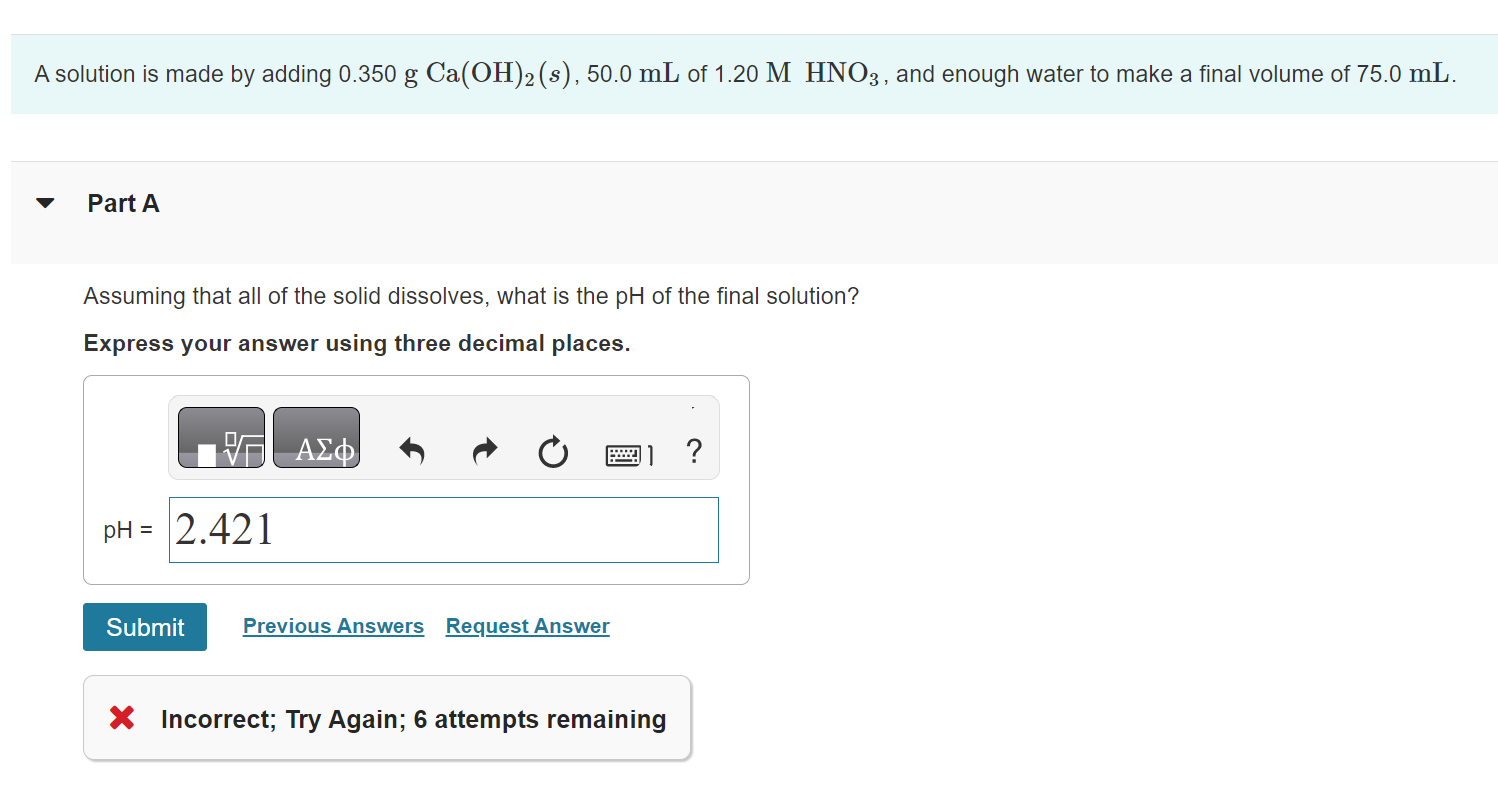
A solution is made by adding 0.350gCa(OH)2(s),50.0mL of 1.20MHNO3, and enough water to make a final volume of 75.0mL. Part A Assuming that all of the solid dissolves, what is the pH of the final solution? Express your answer using three decimal places. X Incorrect; Try Again; 6 attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


