Question: A stage extraction process is depicted in figure below. In such systems, a stream containing a weight fraction yin of a chemical enters from the
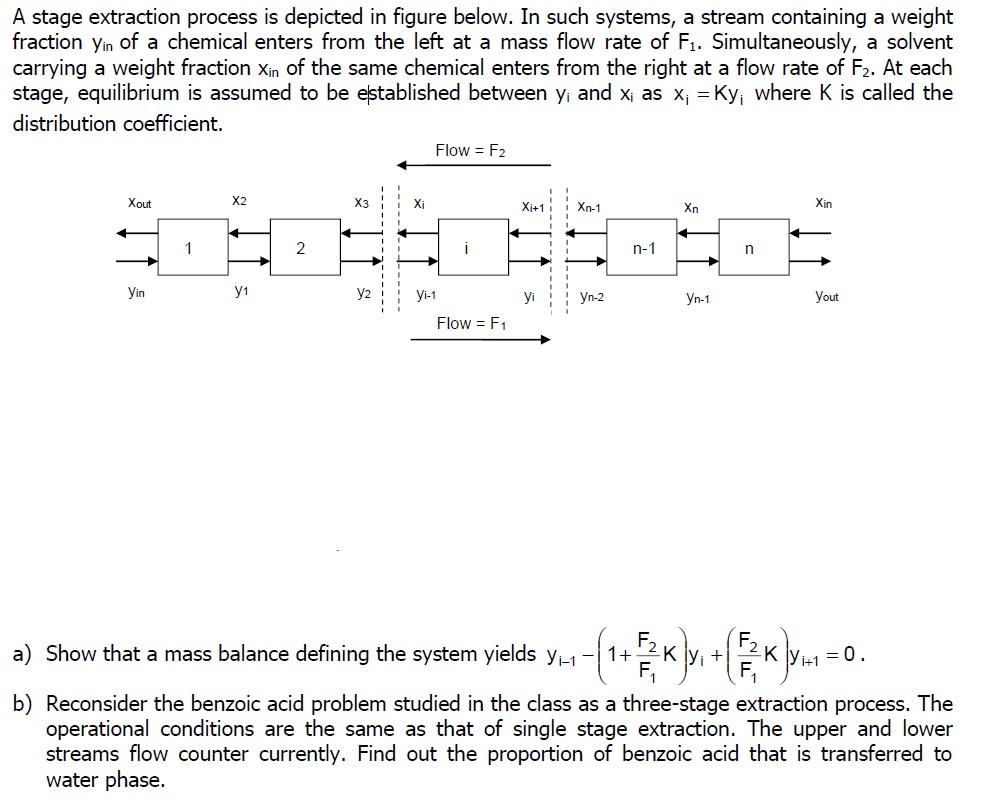
A stage extraction process is depicted in figure below. In such systems, a stream containing a weight fraction yin of a chemical enters from the left a mass flow rate of F1. Simultaneously, a solvent carrying a weight fraction Xin of the same chemical enters from the right at a flow rate of F2. At each stage, equilibrium is assumed to be established between yi and xi as Xi = Ky; where K is called the distribution coefficient. Flow = F2 Xout X2 X3 Xi Xi+1 Xn-1 Xn Xin 1 2 n-1 n Yin y1 y2 Yi-1 yi Yn-2 Yn-1 Yout Flow = F1 a) Show that a mass balance defining the system yields Yi-1-1+ --( * * * * * - ly. + 1+1 = 0. b) Reconsider the benzoic acid problem studied in the class as a three-stage extraction process. The operational conditions are the same as that of single stage extraction. The upper and lower streams flow counter currently. Find out the proportion of benzoic acid that is transferred to water phase
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


