Question: Problem 2: 9.13 A stage extraction process is depicted in Fig. P9.13. In such systems, a stream containing a weight fraction y., of a chemical

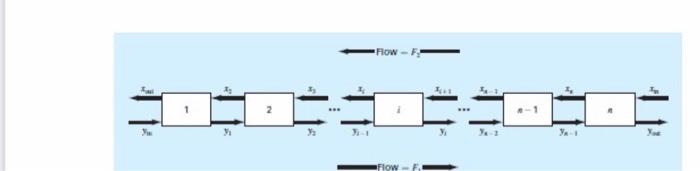
Problem 2: 9.13 A stage extraction process is depicted in Fig. P9.13. In such systems, a stream containing a weight fraction y., of a chemical enters from the left at a mass flow rate of F. Simultaneously, a solvent carrying a weight fraction - of the same chemical enters from the right at a flow rate of F2. Thus, for stage i, a mass balance can be represented as F1X FY-1+F2X1+1 = F/y2 + F2X (P9.13a) At each stage, an equilibrium is assumed to be established between y, and, as in K (P9.136) where K is called a distribution coefficient. Equation (P9.135) can be solved for x; and substituted into Eq. (P9.13a) to yield 9-1-1 - (1+**)>+() Y 42+1 (P9.130) If F = 400 kg/h, Yin = 0.1, F2 = 800 kg/h, x = 0, and K = 5, determine the values of you and out if a five-stage reactor is used. Note that Eq. (P9.13c) must be modified to account for the inflow weight fractions when applied to the first and last stages. -Flow- 2 * Flow
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


