Question: Above what Fe2+ concentration will Fe(OH)2 precipitate from a buffer solution that has a pH of 8.67 ? The Ksp of Fe(OH)2 is 4.871017
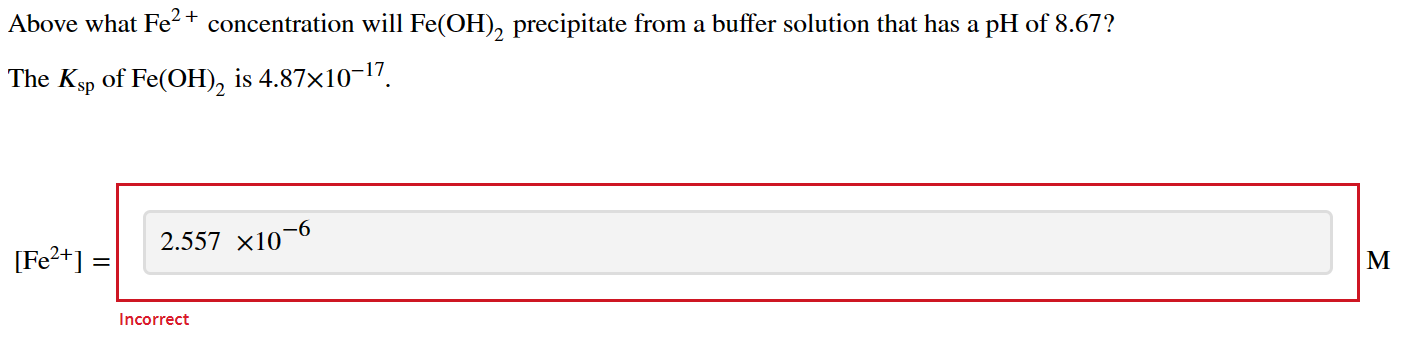
Above what Fe2+ concentration will Fe(OH)2 precipitate from a buffer solution that has a pH of 8.67 ? The Ksp of Fe(OH)2 is 4.871017
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


